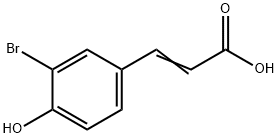
3-BROMO-4-HYDROXYCINNAMIC ACID synthesis
- Product Name:3-BROMO-4-HYDROXYCINNAMIC ACID
- CAS Number:119405-32-2
- Molecular formula:C9H7BrO3
- Molecular Weight:243.05
Yield: 75%
Reaction Conditions:
with piperidine;pyridine at 80; for 5 h;
Steps:
4.2. Synthesis of 3-Br-pCA
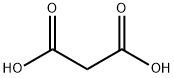
201 mg (1 mmol) 3-bromo-4-hydroxybenzaldehyde (Sigma-Aldrich) and 229 mg (2.2 mmol) malonic acid (Sigma-Aldrich) was added to a 10 mL round-bottom flask containing a stir bar. 450 μL pyridine (J.T.Baker) and 18 μL piperidine (Sigma-Aldrich) was then added with stirring. The flask was submerged in a water bath held at 80 °C and refluxed for 5 h. The flask was then cooled to room temperature before dropwise addition of 9 mL 2M hydrochloric acid to produce a white precipitate. The solid was isolated with vacuum filtration, washed with additional 2M hydrochloric acid, and dried completely under vacuum, producing 181 mg of the desired 3-Br-pCA product (75 % yield). Any residual water in the product will prevent proper subsequent chromophore functionalization. LC-MS and 1H NMR data confirmed theidentity and purity (Fig. S1). The residual pyridinium salt detected in the 1H NMR spectrum does not interfere with any downstream reactions.
References:
Boxer, Steven G.;Lin, Chi-Yun;Romei, Matthew G. [Journal of Photochemistry and Photobiology A: Chemistry,2020,vol. 401]
73747-61-2
0 suppliers
inquiry

119405-32-2
11 suppliers
inquiry
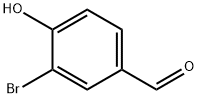
2973-78-6
224 suppliers
$14.00/5g

119405-32-2
11 suppliers
inquiry
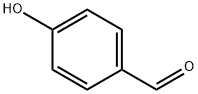
123-08-0
961 suppliers
$5.00/10g

119405-32-2
11 suppliers
inquiry