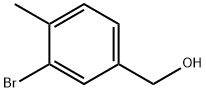
(3-BroMo-4-Methylphenyl)Methanol synthesis
- Product Name:(3-BroMo-4-Methylphenyl)Methanol
- CAS Number:68120-35-4
- Molecular formula:C8H9BrO
- Molecular Weight:201.06
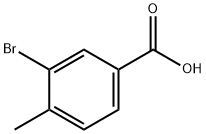
7697-26-9

68120-35-4
GENERAL METHODS: 3-Bromo-4-methylbenzoic acid (20.70 g, 81.82 mmol) was dissolved in tetrahydrofuran (THF, 250 mL) and the solution was cooled to 0°C. Borane-THF complex (1.0 M in THF, 164 mL) was slowly added under stirring. The reaction mixture was gradually warmed to room temperature and stirring was continued for 2 hours. Upon completion of the reaction, the reaction was quenched with saturated sodium carbonate solution (Na2CO3, 100 mL). Subsequently, the reaction mixture was extracted with ethyl acetate (EtOAc, 3 x 100 mL). The organic layers were combined, dried with anhydrous magnesium sulfate (MgSO4), filtered and concentrated under reduced pressure to give the crude product as a colorless liquid. The crude product was further purified by distillation under reduced pressure (0.8 mbar) and the fractions distilled at 90 °C were collected to give 3-bromo-4-methylbenzyl alcohol (16.30 g, 99%) as a colorless liquid.
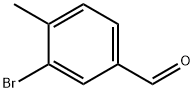
36276-24-1
129 suppliers
$31.00/1g

68120-35-4
75 suppliers
$15.00/250mg
Yield: 49.6%
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0 - 20; for 2 h;
Steps:
Intermediate 4b: (3-Bromo-4-methylphenyl) methanol
To a stirred solution of 3-bromo-4-methylbenzaldehyde (10.0 g, 50.2 mmol) in dry methanol (60 mL) at 0 00 NaBH4 (2.3 g, 36.0 mmol) was added portion wise and theresulting solution was stirred at room temperature for 2 h. The reaction mixture was quenched with ice water, extracted with ethyl acetate; the organic layer was washed with water and brine solution, dried over anhydrous Na2SO4 and concentrated. The product was purified by column chromatography to yield title compound (5.0 g, 49.60%) as yellow oil.
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;MADANAHALLI RANGANATH RAO, Jagannath;GURRAM RANGA, Madhavan;PACHIYAPPAN, Shanmugam WO2014/202580, 2014, A1 Location in patent:Page/Page column 77; 78

7697-26-9
265 suppliers
$6.00/5g

68120-35-4
75 suppliers
$15.00/250mg
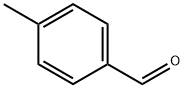
104-87-0
518 suppliers
$6.00/25g

68120-35-4
75 suppliers
$15.00/250mg