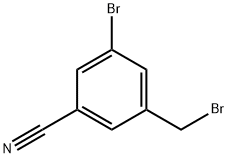
3-Bromo-5-cyanobenzyl bromide synthesis
- Product Name:3-Bromo-5-cyanobenzyl bromide
- CAS Number:124289-24-3
- Molecular formula:C8H5Br2N
- Molecular Weight:274.94
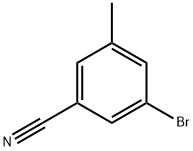
124289-21-0
114 suppliers
$8.00/100mg

124289-24-3
33 suppliers
inquiry
Yield: 53%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane at 80; for 8 h;
Steps:
44.1 Step 1: Example 44b
To a solution of Example 44a (11.0 g, 56.1 mmol, 1.0 eq) in CCl4 (500 mL) were added NBS (15.0 g, 84.2 mmol, 1.5 eq) and AIBN (4.6 g, 28.1 mmol, 0.5 eq). The reaction mixture was stirred at 80°C for 8 h. After the insoluble solid was removed, the filtrate was concentrated, and the residue was purified by silica gel flash column chromatography to afford the product Example 44b (8.1 g, 53% yield) as a yellow solid. LCMS[M+1] + = 275.9.
References:
FRONTHERA U.S. PHARMACEUTICALS LLC;JIN, Bohan;DONG, Qing;HUNG, Gene WO2020/185755, 2020, A1 Location in patent:Paragraph 00552
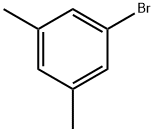
556-96-7
382 suppliers
$6.00/5g

124289-24-3
33 suppliers
inquiry
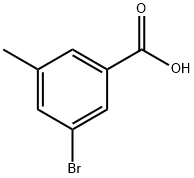
58530-13-5
125 suppliers
$8.00/1g

124289-24-3
33 suppliers
inquiry
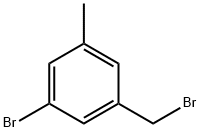
51719-69-8
27 suppliers
$87.00/250mg

124289-24-3
33 suppliers
inquiry
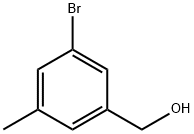
648439-19-4
52 suppliers
$45.00/100mg

124289-24-3
33 suppliers
inquiry