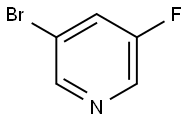
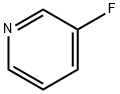
3-Bromo-5-fluoropyridine synthesis
- Product Name:3-Bromo-5-fluoropyridine
- CAS Number:407-20-5
- Molecular formula:C5H3BrFN
- Molecular Weight:175.99
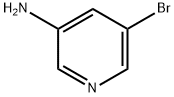
13535-01-8

407-20-5
Step 2: Synthesis of 3-bromo-5-fluoropyridine To a cooled solution of 10.0 g (0.058 mol) of 3-amino-5-bromopyridine dissolved in 59 mL of 50% tetrafluoroboric acid was added dropwise at -10 °C a solution prepared from 4.19 g (0.06 mol) of sodium nitrite dissolved in 13 mL of water. The reaction mixture was stirred at -8°C for 1 hr and then 150 mL of ether was added. The crude diazonium salt was collected by filtration and washed with ether. Subsequently, the crude diazonium salt was added in batches to 200 mL of toluene preheated to 80°C. The reaction mixture was stirred at 90°C for 1 hour and then the organic phase was concentrated. The light yellow residue was suspended in 150 mL of water and the pH was adjusted with 32% sodium hydroxide solution to 11. The aqueous phase was extracted three times with 200 mL of dichloromethane. The organic phases were combined, washed with water, dried over magnesium sulfate, and concentrated. The crude product was purified by vacuum distillation (10 mbar, 35 °C) to give 15.4 g of brown oil, which was further purified to give 5.6 g (0.032 mol, 55% yield) of the target compound 3-bromo-5-fluoropyridine as a colorless oil (ISP): m/e = 176.1, 178.1 (M + H+).

13535-01-8
384 suppliers
$3.00/1g

407-20-5
348 suppliers
$10.00/1g
Yield:407-20-5 55%
Reaction Conditions:
with tetrafluoroboric acid;sodium nitrite in water at -8; for 1 h;Sandmeyer Reaction;
Steps:
34.2
Step 2: 3-Bromo-5-fluoropyridine A at -10° C. cooled solution of 10.0 g (0.058 mol) of 3-Amino-5-bromopyridine in 59 ml of 50% Tetrafluoroboric acid was treated by dropwise addition of a solution of 4.19 g (0.06 mol) of sodium nitrite in 13 ml of water. After stirring for 1 h at -8° C., 150 ml of ether was added to the brown suspension. The crude diazonium salt was filtered off, and washed with ether. This crude salt was then added in portions to 200 ml of toluene heated at 80° C. After stirring for 1 h at 90° C., the organic phase was concentrated. The light yellow residue was suspended in 150 ml of water and the pH was adjusted to 11 with 32% sodium hydroxide solution. The resulting solution was extracted three times with 200 ml of methylene chloride. The combined organic phases were washed with water, dried over magnesium sulfate and concentrated. The crude material 15.4 g (brown oil) was purified by vaccum distillation (10 mBar, 35° C.) and yielded 5.6 g (0.032 mol, 55%) of the title compound as a colorless oil (ISP): m/e=176.1, 178.1 (M+H+).
References:
Jaeschke, Georg;Kolczewski, Sabine;Porter, Richard Hugh Philip;Vieira, Eric US2006/199960, 2006, A1 Location in patent:Page/Page column 21
625-92-3
488 suppliers
$6.00/10g

407-20-5
348 suppliers
$10.00/1g
2546-52-3
176 suppliers
$24.00/250mg
372-47-4
383 suppliers
$5.00/1g

407-20-5
348 suppliers
$10.00/1g