
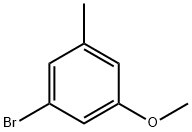
3-BROMO-5-METHOXYBENZALDEHYDE synthesis
- Product Name:3-BROMO-5-METHOXYBENZALDEHYDE
- CAS Number:262450-65-7
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
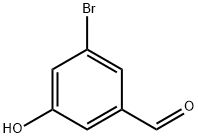
199177-26-9

74-88-4

262450-65-7
Example 1 5A: Synthesis of 3-bromo-5-methoxybenzaldehyde 3-Bromo-5-hydroxybenzaldehyde (0.5 g, 2.487 mmol) was dissolved in N,N-dimethylformamide (DMF, 14.63 mL) and the solution was cooled to 0 °C. Under stirring, sodium hydride (NaH, 0.119 g, 4.97 mmol) was added in three batches. Subsequently, the reaction mixture was slowly warmed to room temperature and iodomethane (MeI, 0.933 mL, 14.92 mmol) was added. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the reaction mixture was diluted with water and partially concentrated under reduced pressure. The concentrate was diluted with dichloromethane (DCM), washed twice sequentially with water and once with saturated brine, and then dried over anhydrous sodium sulfate (Na2SO4). After filtration to remove the desiccant, the filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography with an elution gradient of 0 to 100% ethyl acetate (EtOAc) in hexane solution to give the title compound Example 15A (0.488 g, 2.27 mmol, 9% yield). 1H NMR (400 MHz, chloroform-d) δ 9.91 (1H, s), 7.58 (1H, t, J = 1.38 Hz), 7.28-7.35 (2H, m), 3.86 (3H, s).

199177-26-9
89 suppliers
$23.00/250mg

74-88-4
357 suppliers
$15.00/10g

262450-65-7
123 suppliers
$6.00/100mg
Yield: 91%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 20;
Steps:
15.15A Example 1 5A: 3 -Bromo-5-methoxybenzaldehyde
Example 1 5A: 3 -Bromo-5-methoxybenzaldehyde j00295j 3-Bromo-5-hydroxybenzaldehyde (0.5 g, 2.487 mmol) was dissolved in DMF(14.63 mL) and cooled to 0 °C. NaH (0.119 g, 4.97 mmol) was added in three portions.The flask was immediately allowed to warm to ambient temperature and Mel (0.93 3 mL,14.92 mmol) was added, and the reaction stirred overnight. The reaction was diluted with water and partially concentrated in vacuo. The material was diluted with DCM and washed twice with water, washed with brine, dried (Na2SO4), filtered, and concentrated in vacuo. The crude material was purified by silica gel column chromatography (gradientfrom 0 to 100% EtOAc in hexanes) to yield Example 15A (0.488 g, 2.27 mmol, 9 1%).‘H NMR (400 MHz, CHLOROFORM-cl) ö ppm 9.91 (1 H, s), 7.58 (1 H, t, J=1.38 Hz), 7.28 - 7.35 (2 H, m, J=2.38, 2.13, 2.01, 2.01 Hz), 3.86 (3 H, s).
References:
BRISTOL-MYERS SQUIBB COMPANY;RICHTER, Jeremy;BATES, J. Alex;CHENEY, Daniel L. WO2014/201073, 2014, A1 Location in patent:Paragraph 00295

60-29-7
0 suppliers
$30.00/50g
74137-36-3
221 suppliers
$8.00/1g

262450-65-7
123 suppliers
$6.00/100mg
262450-64-6
48 suppliers
$62.50/1 g

262450-65-7
123 suppliers
$6.00/100mg
157893-14-6
122 suppliers
$10.00/250mg

262450-65-7
123 suppliers
$6.00/100mg
29578-83-4
151 suppliers
$6.00/1g

262450-65-7
123 suppliers
$6.00/100mg