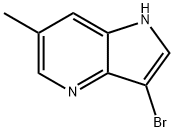
3-BroMo-6-Methyl-4-azaindole synthesis
- Product Name:3-BroMo-6-Methyl-4-azaindole
- CAS Number:1190323-01-3
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
1175015-76-5
52 suppliers
$45.00/10mg

1190323-01-3
5 suppliers
inquiry
Yield:1190323-01-3 41%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0; for 1 h;Inert atmosphere;Schlenk technique;
Steps:
Step1: 3-Bromo-1H-pyrrolo[3,2-b]pyridine:
General procedure: To a stirredsolution of 1H-pyrrolo[3,2-b]pyridine(1.0 g, 8.47 mmol) in dry DMF (10 ml) was added N-bromosuccinimide(1.51 g, 8.48 mmol) at 0 °C and the resultant mixture was stirredfor 1 h at the same temperature. The reaction mixture was dilutedwith saturated sodium bicarbonate solution (50 ml) and extracted withethyl acetate (2 x 150 ml). The combined organic layers were washedwith brine (50 ml) and dried (Na2SO4).The residue obtained after evaporation of the solvent was purified bysilica gel column chromatography using3% methanol in chloroformm toyield 1.12 g (67%) of product as a white solid.
References:
Das, Sanjib;Shelke, Dnyaneshwar E.;Harde, Rajendra L.;Avhad, Vijayshree B.;Khairatkar-Joshi, Neelima;Gullapalli, Srinivas;Gupta, Praveen K.;Gandhi, Maulik N.;Bhateja, Deepak K.;Bajpai, Malini;Joshi, Ashwini A.;Marathe, Megha Y.;Gudi, Girish S.;Jadhav, Satyawan B.;Mahat, Mahamad Yunnus A.;Thomas, Abraham [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 15,p. 3238 - 3242] Location in patent:supporting information