3-BROMO-8-CHLOROIMIDAZO[1,2-A]PYRAZINE synthesis
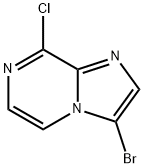
- Product Name:3-BROMO-8-CHLOROIMIDAZO[1,2-A]PYRAZINE
- CAS Number:143591-61-1
- Molecular formula:C6H3BrClN3
- Molecular Weight:232.47
![8-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/69214-33-1.gif)
69214-33-1
![3-BROMO-8-CHLOROIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/143591-61-1.gif)
143591-61-1
General procedure for the synthesis of 3-bromo-8-chloroimidazo[1,2-a]pyrazine from 8-chloroimidazo[1,2-a]pyrazine: N-bromosuccinimide (1.78 g, 10 mmol) was added to a solution of 8-chloroimidazo[1,2-a]pyrazine (1.53 g, 10 mmol) in dichloromethane (30 mL), and the reaction was stirred for 2 hours at room temperature. After the reaction was completed, the reaction mixture was washed with saturated aqueous sodium carbonate solution (2×20 mL), dried with anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give 3-bromo-8-chloroimidazo[1,2-a]pyrazine (2.11 g, 96% yield).
![8-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/69214-33-1.gif)
69214-33-1
151 suppliers
$18.00/250mg
![3-BROMO-8-CHLOROIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/143591-61-1.gif)
143591-61-1
132 suppliers
$14.00/250mg
Yield:143591-61-1 96%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 20; for 2 h;
Steps:
To a solution of 8-chloro-imidazo[l,2-a]pyrazine (1.53 g, 0.01 mol) in DCM (30 mL) is added N-bromosuccinimide (1.78 g, 0.01 mol) and the reaction is stirred at room temperature for 2h. After this time the solution is washed with saturated aqueous solution of Na2CO3 (2 x 20 mL), dried (MgSO4), filtered and concentrated in vacuo to give 3-bromo-8-chloro-imidazo[l,2- a]pyrazine (2.11 g, 96%).
References:
BIOFOCUS DPI LIMITED WO2009/24585, 2009, A2 Location in patent:Page/Page column 46
![8-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/69214-33-1.gif)
69214-33-1
151 suppliers
$18.00/250mg
![3-BROMO-8-CHLOROIMIDAZO[1,2-A]PYRAZINE](/CAS/GIF/143591-61-1.gif)
143591-61-1
132 suppliers
$14.00/250mg
![3,8-Dibromoimidazo[1,2-a]pyrazine](/CAS/20150408/GIF/936361-36-3.gif)
936361-36-3
11 suppliers
inquiry