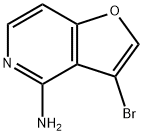
3-bromofuro[3,2-c]pyridin-4-amine synthesis
- Product Name:3-bromofuro[3,2-c]pyridin-4-amine
- CAS Number:799293-73-5
- Molecular formula:C7H5BrN2O
- Molecular Weight:213.03
![3-Bromo-4-chlorofuro[3,2-c]pyridine](/CAS/GIF/220939-72-0.gif)
220939-72-0
![3-bromofuro[3,2-c]pyridin-4-amine](/CAS/GIF/799293-73-5.gif)
799293-73-5
The general procedure for the synthesis of 3-bromofuro[3,2-c]pyridin-4-amine using 3-bromo-4-chlorofuro[3,2-c]pyridine as starting material was as follows: 200 mg of 3-bromo-4-chlorofuro[3,2-c]pyridine, 3 mL of concentrated ammonia and 3 mL of dioxane were added to a stainless steel sealing tube, which was sealed and then heated to 150 °C. The reaction was carried out with continuous stirring. After 3 days of reaction under continuous stirring, the reaction mixture was cooled to room temperature. Subsequently, water and ethyl acetate were added to the mixture for liquid-liquid separation. The aqueous phase was further extracted with ethyl acetate. All organic phases were combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated to dryness to afford 98 mg of 3-bromofuro[3,2-c]pyridin-4-amine as a brown solid in 67% yield. The product was characterized by 1H NMR (300 MHz, DMSO-d6) with chemical shifts δ (ppm) of 6.19 (s, 2H), 6.92 (d, J = 6.0 Hz, 1H), 7.85 (d, J = 6.0 Hz, 1H), 8.11 (s, 1H).
![3-Bromo-4-chlorofuro[3,2-c]pyridine](/CAS/GIF/220939-72-0.gif)
220939-72-0
42 suppliers
$62.00/250mg
![3-bromofuro[3,2-c]pyridin-4-amine](/CAS/GIF/799293-73-5.gif)
799293-73-5
33 suppliers
$160.00/250mg
Yield:799293-73-5 67%
Reaction Conditions:
with ammonium hydroxide in 1,4-dioxane;water at 150; for 72 h;
References:
Lv, Yongcong;Li, Mengyuan;Liu, Ting;Tong, Linjiang;Peng, Ting;Wei, Lixin;Ding, Jian;Xie, Hua;Duan, Wenhu [ACS Medicinal Chemistry Letters,2014,vol. 5,# 5,p. 592 - 597] Location in patent:supporting information

59898-36-1
0 suppliers
inquiry
![3-bromofuro[3,2-c]pyridin-4-amine](/CAS/GIF/799293-73-5.gif)
799293-73-5
33 suppliers
$160.00/250mg
![4,5-DIHYDRO-4-OXOFURO[3,2-C]PYRIDINE](/CAS/GIF/26956-43-4.gif)
26956-43-4
112 suppliers
$10.00/100mg
![3-bromofuro[3,2-c]pyridin-4-amine](/CAS/GIF/799293-73-5.gif)
799293-73-5
33 suppliers
$160.00/250mg
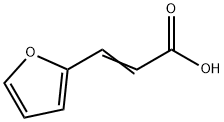
539-47-9
273 suppliers
$10.00/1g
![3-bromofuro[3,2-c]pyridin-4-amine](/CAS/GIF/799293-73-5.gif)
799293-73-5
33 suppliers
$160.00/250mg
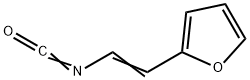
29080-01-1
0 suppliers
inquiry
![3-bromofuro[3,2-c]pyridin-4-amine](/CAS/GIF/799293-73-5.gif)
799293-73-5
33 suppliers
$160.00/250mg