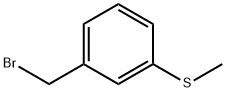
(3-(bromomethyl)phenyl)(methyl)sulfane synthesis
- Product Name:(3-(bromomethyl)phenyl)(methyl)sulfane
- CAS Number:90532-01-7
- Molecular formula:C8H9BrS
- Molecular Weight:217.13
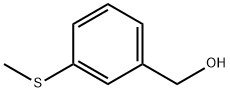
59083-33-9
10 suppliers
$265.00/100mg

90532-01-7
5 suppliers
inquiry
Yield:90532-01-7 46%
Reaction Conditions:
with hydrogen bromide in diethyl ether at 0 - 20; for 19.17 h;
Steps:
10
Hydrogen bromide gas was bubbled through a solution of the alcohol of Preparation 9 (568mg, 3.68mmol) in diethyl ether (30ml) at 0°C for one hour. The reaction mixture was then warmed to room temperature and stirred for 30 minutes. TLC showed that the reaction was not complete. The reaction mixture was saturated again with hydrogen bromide gas for fortyminutes at O0C and then stirred at room temperature for eighteen hours. Nitrogen gas was then bubbled through the reaction to remove any remaining hydrogen bromide gas. The reaction mixture was concentrated under reduced pressure. The crude product EPO
References:
WO2006/120544,2006,A1 Location in patent:Page/Page column 49-50
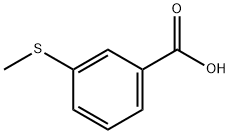
825-99-0
102 suppliers
$17.00/1g

90532-01-7
5 suppliers
inquiry