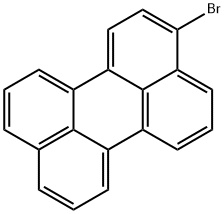
3-BroMoperylene synthesis
- Product Name:3-BroMoperylene
- CAS Number:23683-68-3
- Molecular formula:C20H11Br
- Molecular Weight:331.21
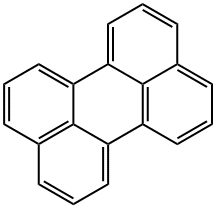
198-55-0

23683-68-3
General procedure for the synthesis of 3-bromo perylene from perylene: 1. 3.0 g (11 mmol) of perylene was dissolved in 700 mL of dichloromethane and stirred for 5 minutes until completely dissolved. 2. 2.11 g (12 mmol, 1.1 eq.) of N-bromosuccinimide was slowly added dropwise to the above solution at room temperature. 3. After the dropwise addition, the reaction mixture was stirred at room temperature overnight. 4. 4. Upon completion of the reaction, the reaction solution was purified by silica gel column chromatography with the unfolding reagent ratio of dichloromethane:hexane=1:1 (v/v) to remove unreacted N-bromosuccinimide. 5. Collect the target component and concentrate the solvent under reduced pressure to obtain yellow crystalline 3-bromhydrazone in 90% yield. 6. The structure of the product was confirmed by 1H-NMR and ESI-MS. - 1H-NMR (CDCl3, 400MHz) δ: 7.46-7.51 (m, 2H), 7.59 (t, J=4.4Hz, 1H), 7.68-7.72 (m, 2H), 7.78-7.80 (m, 1H), 7.98-8.04 (m, 1H), 8.09-8.14 (m, 1H), 8.17- 8.28 (m, 3H). 8.28 (m, 3H). - ESI-MS (C20H11Br): Calculated: 330.00; measured: 331.9 [M+H]+.

198-55-0
258 suppliers
$6.00/1g
85514-20-1
7 suppliers
inquiry
56752-35-3
56 suppliers
inquiry

23683-68-3
80 suppliers
$44.00/100mg
Yield: 90%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 20;
Steps:
Mixture of Brominated Perylenes
To a suspension of perylene (25.0 g, 100 mmol, 1 equiv) in DMF (1000 mL) a solution of NBS (23.0 g, 130 mmol, 1.3 equiv) in DMF (400 mL) was added via a syringe pump for 10 h. The obtained mixture was stirred overnight at r.t. and poured into water (2000 mL). The precipitate obtained was filtered off on a glass frit (G3) and was subsequently washed with EtOH (100 mL), Et2O (100 mL), and finally with CH2Cl2 (3 × 100 mL). The obtained solid was dried under vacuum, yielding a mixture of brominated perylenes; yield: 29.8 g (90%); 89% of 3-bromoperylene by GC-MS; yellow solid.
References:
Guzeev, Bogdan A.;Mladentsev, Dmitry Y.;Sharikov, Mikhail I.;Goryunov, Georgy P.;Uborsky, Dmitry V.;Voskoboynikov, Alexander Z. [Synthesis,2019,vol. 51,# 6,p. 1399 - 1407]

198-55-0
258 suppliers
$6.00/1g

23683-68-3
80 suppliers
$44.00/100mg