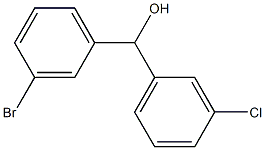
(3-bromophenyl)-(3-chlorophenyl)methanol synthesis
- Product Name:(3-bromophenyl)-(3-chlorophenyl)methanol
- CAS Number:1093865-36-1
- Molecular formula:C13H10BrClO
- Molecular Weight:297.5749
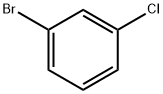
108-37-2
357 suppliers
$6.00/10g
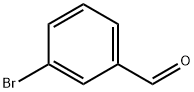
3132-99-8
478 suppliers
$5.00/10g

1093865-36-1
5 suppliers
$437.00/1g
Yield:1093865-36-1 52%
Reaction Conditions:
Stage #1: 1-bromo-3-chlorobenzenewith n-butyllithium in tetrahydrofuran at -78; for 1.33333 h;
Stage #2: m-bromobenzoic aldehyde in tetrahydrofuran at -78; for 0.166667 h;
Stage #3: with ammonia hydrochloride in tetrahydrofuran;lithium hydroxide monohydrate at -78 - 20;
Steps:
19.1
A solution of l-bromo-3-chlorobenzene (20 g, 104 mmol) in anhydrous THF (125 mL) under N2 was cooled to -78 0C, ?-BuLi (2.5 M, 42 mL, 105 mmol) was then added drop wise over 20 min. After stirring an additional hour, 3- bromobenzaldehyde (12.5 mL, 104 mmol) was added drop wise and the reaction was stirred for another 10 min at -78 0C. Sat. NH4Cl (100 mL) was added and the reaction was warmed to room temperature and extracted with ether (300 mL). The ether layer was extracted with three 75 mL portions of 1 M sodium bisulfite, 75 mL of 1 M sodium hydroxide, 75 mL of water, and 75 mL of brine. The ether layer was dried over MgSO4 and the solvent removed to give crude product, which was purified by chromatography on silica gel (16 g, 52%). 1H NMR (CDCl3) δ 2.27 (br, IH), 5.75 (s, IH), 7.14-7.54 (m, 8H).
References:
WO2008/156817,2008,A2 Location in patent:Page/Page column 123-124