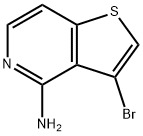
3-bromothieno[3,2-c]pyridin-4-amine synthesis
- Product Name:3-bromothieno[3,2-c]pyridin-4-amine
- CAS Number:799293-85-9
- Molecular formula:C7H5BrN2S
- Molecular Weight:229.1
![3-BROMO-4-CHLOROTHIENO[3,2-C]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB5449945.gif)
29064-82-2
![3-bromothieno[3,2-c]pyridin-4-amine](/CAS/GIF/799293-85-9.gif)
799293-85-9
The general procedure for the synthesis of 3-bromothieno[3,2-c]pyridin-4-amine from 3-bromo-4-chlorothieno[3,2-c]pyridine is as follows: 3-bromo-4-chlorothieno[3,2-c]pyridine (3 g, 12 mmol, prepared according to the method described in Bull.Soc.Chim.Belges 1970,79,407-414) was mixed with concentrated ammonia (100 mL) and p-dioxane (100 mL) was mixed, sealed in a stainless steel high-pressure reactor, and the reaction was stirred at 150°C for 18 hours. Upon completion of the reaction, the mixture was concentrated to half the original volume, diluted with water and extracted with ethyl acetate. The organic extracts were combined, washed with brine, dried over anhydrous magnesium sulfate, filtered and concentrated to afford the target product 3-bromothieno[3,2-c]pyridin-4-amine 2.6 g in 94% yield. The product was identified by 1H NMR (DMSO-d6, 400 MHz): δ 7.83 (d, 1H), 7.77 (s, 1H), 7.26 (d, 1H), 6.48 (br s, 2H); mass spectrum (MS) m/e 229 (M + H)+.
![3-BROMO-4-CHLOROTHIENO[3,2-C]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB5449945.gif)
29064-82-2
66 suppliers
$45.00/50mg
![3-bromothieno[3,2-c]pyridin-4-amine](/CAS/GIF/799293-85-9.gif)
799293-85-9
83 suppliers
$51.00/100mg
Yield:799293-85-9 94%
Reaction Conditions:
with ammonia in 1,4-dioxane;water at 150; for 18 h;
Steps:
219.A EXAMPLE 219; 3-(4-phenoxyphenyl)thieno[3,2-c]pyridin-4-amine; EXAMPLE 219A; 3-bromothieno[3,2-c]pyridin-4-amine
A mixture of 3-bromo-4-chlorothieno[3,2-c]pyridine (prepared according to the procedure described in Bull. Soc. Chim. Belges 1970, 79, 407-414, 3 g, 12 mmol), concentrated aqueous NH4OH (100 mL), and p-dioxane (100 mL) was sealed in a stainless steel, high-pressure reactor and stirred for 18 hours at 150 C. The mixture was concentrated to half its original volume, diluted with water, and extracted with ethyl acetate. The combined organic extracts were washed with brine, dried (MgSO4), filtered, and concentrated to provide 2.6 g (94%) of the desired product. 1H NMR (DMSO-d6, 400 MHz) ? 7.83 (d, 1H), 7.77 (s, 1H), 7.26 (d, 1H), 6.48 (br s, 2H); MS m/e 229 (M+H)+.
References:
Betschmann, Patrick;Burchat, Andrew;Calderwood, David;Curtin, Michael L.;Davidsen, Steven K.;Davis, Heather M.;Frey, Robin R.;Heyman, Howard R.;Hirst, Gavin;Hrnciar, Peter;Michaelides, Michael;Rafferty, Paul US2005/20619, 2005, A1 Location in patent:Page 47
![3-broMo-4H,5H-thieno[3,2-c]pyridin-4-one](/CAS/GIF/799293-83-7.gif)
799293-83-7
81 suppliers
$45.00/100mg
![3-bromothieno[3,2-c]pyridin-4-amine](/CAS/GIF/799293-85-9.gif)
799293-85-9
83 suppliers
$51.00/100mg
![3-BROMO-4-CHLOROTHIENO[3,2-C]PYRIDINE](/StructureFile/ChemBookStructure9/GIF/CB5449945.gif)
29064-82-2
66 suppliers
$45.00/50mg
![3-broMo-4H,5H-thieno[3,2-c]pyridin-4-one](/CAS/GIF/799293-83-7.gif)
799293-83-7
81 suppliers
$45.00/100mg
![3-bromothieno[3,2-c]pyridin-4-amine](/CAS/GIF/799293-85-9.gif)
799293-85-9
83 suppliers
$51.00/100mg
![3-broMo-4H,5H-thieno[3,2-c]pyridin-4-one](/CAS/GIF/799293-83-7.gif)
799293-83-7
81 suppliers
$45.00/100mg
![3-bromothieno[3,2-c]pyridin-4-amine](/CAS/GIF/799293-85-9.gif)
799293-85-9
83 suppliers
$51.00/100mg
18791-75-8
287 suppliers
$9.00/5g
![3-bromothieno[3,2-c]pyridin-4-amine](/CAS/GIF/799293-85-9.gif)
799293-85-9
83 suppliers
$51.00/100mg