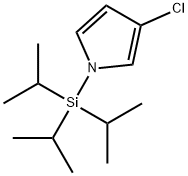
3-Chloro-1-[tris(1-methylethyl)silyl]-1H-pyrrole synthesis
- Product Name:3-Chloro-1-[tris(1-methylethyl)silyl]-1H-pyrrole
- CAS Number:130408-83-2
- Molecular formula:C13H24ClNSi
- Molecular Weight:257.87
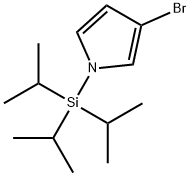
87630-36-2
123 suppliers
$17.00/250mg
![3-Chloro-1-[tris(1-methylethyl)silyl]-1H-pyrrole](/CAS/GIF/130408-83-2.gif)
130408-83-2
14 suppliers
inquiry
Yield:130408-83-2 77%
Reaction Conditions:
Stage #1: 3-bromo-1-triisopropylsilanyl-1H-pyrrolewith tert.-butyl lithium in tetrahydrofuran;pentane at -78; for 0.5 h;
Stage #2: with hexachloroethane in tetrahydrofuran;pentane at -78; for 0.5 h;
Steps:
c c) 3-Chloro-1 -(triisopropylsilyl)-l H-pyrrole
o a solution of 3-bromo-1-(triisopropylsilyl)-1 H-pyrrole (Intermediate 28b, 1.0 g, 3.31 mmol) in THF (6.5 ml_) at -78 °C was added dropwise te/f-butyllithium (1.7M solution in pentane, 3.99 ml_, 6.8 mmol) and the mixture was stirred at -78 °C for 30 min. A solution of perchloroethane (1.57 g, 6.62 mmol) in THF (10 ml_) was added dropwise and the reaction mixture was stirred for 30 min at -78 °C and then allowed to warm to room temperature. Aqueous saturated ammonium chloride solution was added, phases were separated and the aqueous phase was extracted with EtOAc (x2). The combined organic phases were washed with brine, dried over magnesium sulfate, filtered and the solvent was evaporated to dryness. The residue was purified by flash chromatography (hexanes/diethyl ether) to yield the title compound (654 mg, 77%) as a slightly yellow oil.MS (m/z): 258/260 [M+1/M+3]1H-NMR d (400 MHz, CDCIs): 1.09 (d, J=7 Hz, 18H), 1.41 (dt, J=15 and 7 Hz, 3H), 6.23 (d, J=4 Hz, 1 H), 6.67 (d, J=9 Hz, 2H)
References:
WO2020/245291,2020,A1 Location in patent:Page/Page column 48-49
87630-35-1
155 suppliers
$7.00/250mg
![3-Chloro-1-[tris(1-methylethyl)silyl]-1H-pyrrole](/CAS/GIF/130408-83-2.gif)
130408-83-2
14 suppliers
inquiry
13154-24-0
412 suppliers
$20.00/10g
![3-Chloro-1-[tris(1-methylethyl)silyl]-1H-pyrrole](/CAS/GIF/130408-83-2.gif)
130408-83-2
14 suppliers
inquiry