3-CHLORO-4-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE synthesis
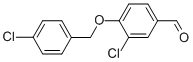
- Product Name:3-CHLORO-4-[(4-CHLOROBENZYL)OXY]BENZALDEHYDE
- CAS Number:443124-79-6
- Molecular formula:C14H10Cl2O2
- Molecular Weight:281.13
Yield:443124-79-6 76%
Reaction Conditions:
with potassium carbonate in acetonitrile at 150; for 0.166667 h;Microwave;
Steps:
28.1
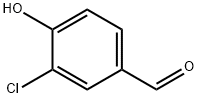
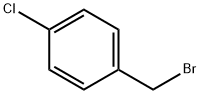
To acetonitrile (15.0 niL) were added 3-chloro-4-hydroxy-benzaldehyde (556, 0.6 g, 4 mmol), 4-chlorobenzyl bromide (557, 1.2 g, 6 mmol), and potassium carbonate (0.9 g, 7 mmol). The reaction was heated to 150 0C for 10 minutes in a CEM Discover microwave instrument. The reaction was poured into water, extracted with ethyl acetate, and washed with brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated. The desired compound was isolated by silica gel column chromatography (ethyl acetate: hexanes) (558, 0.85 g, 76%).
References:
WO2007/2325,2007,A1 Location in patent:Page/Page column 155