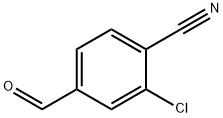
3-Chloro-4-Cyanobenzaldehyde synthesis
- Product Name:3-Chloro-4-Cyanobenzaldehyde
- CAS Number:101048-77-5
- Molecular formula:C8H4ClNO
- Molecular Weight:165.58
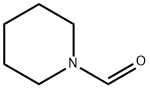
2591-86-8
302 suppliers
$14.00/5g
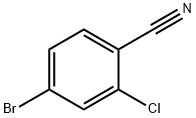
154607-01-9
267 suppliers
$6.00/1g

101048-77-5
27 suppliers
inquiry
Yield: 73.1%
Reaction Conditions:
Stage #1:4-bromo-2-chlorobenzonitrile with isopropylmagnesium chloride in tetrahydrofuran for 1 h;Cooling with ice;
Stage #2:N-Formylpiperidine in tetrahydrofuran for 2 h;Cooling with ice;
Steps:
1.1 Example 1, Preparation of Intermediates I-1 and II-1
(1) Intermediate I-1 (X = Cl): 4-Bromo-2-chlorobenzonitrile (10.0 g, 46.20 mmol) was dissolved in an appropriate amount of 240 mL of anhydrous tetrahydrofuran, Under ice bath conditions, A 2N solution of isopropylmagnesium chloride in tetrahydrofuran (30 ml, 40.06 mmol) was added dropwise. After reaction for 1 h, 3 equivalents of N-formylpiperidine were added under ice-cooling and the reaction was continued for 2 h. The reaction was quenched and extracted with ethyl acetate. The organic layers were combined, washed with water and dried. Column chromatography, eluent: petroleum ether:ethyl acetate=4:1, to obtain 5.6 g of a white solid, yield 73.1%.
References:
Nanjing Tianxiang Pharmaceutical Technology Co., Ltd.;Li Zhenghai;Zhang Aiqin;Tang Lei;Zhang Yongjie;Yang Yong CN107857760, 2018, A Location in patent:Paragraph 0077-0079
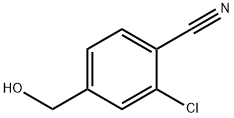
371764-78-2
22 suppliers
inquiry
87413-09-0
526 suppliers
$10.64/1G

101048-77-5
27 suppliers
inquiry