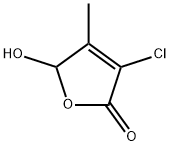
3-chloro-4-methyl-5-hydroxy-2(5H)-furanone synthesis
- Product Name:3-chloro-4-methyl-5-hydroxy-2(5H)-furanone
- CAS Number:112309-61-2
- Molecular formula:C5H5ClO3
- Molecular Weight:148.54
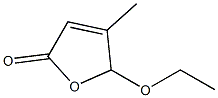
78920-13-5
1 suppliers
inquiry

112309-61-2
2 suppliers
inquiry
Yield:112309-61-2 73%
Reaction Conditions:
with aluminum (III) chloride;chlorine in dichloromethane at 0 - 20; for 6 h;
Steps:
2.2 Procedure for Synthesis of 4-chloro-2-hydroxy-3-methyl-2H-furan-5-one (Step 2)
Procedure for Synthesis of 4-chloro-2-hydroxy-3-methyl-2H-furan-5-one (Step 2)
6 g of 2-ethoxy-3-methyl-2H-furan-5-one (42.20 mmol) was dissolved in dichloromethane (150 mL) and cooled to 0° C.
To this cooled solution aluminium chloride (0.56 g, 4.2 mmol) was added slowly maintaining the temperature of the reaction mixture to 0° C.
Chlorine gas was bubbled into the reaction mixture for 5 h at 0° C. (total weight of the chlorine approximately 12.4 g, 4 eq) and the reaction was brought into room temperature and stirred for 1 h.
Excess chlorine gas was removed by bubbling nitrogen in the reaction mixture, filtered through celite bed and the filtrate was evaporated under vacuum.
The crude mass was dissolved in ethyl acetate (200 mL) and washed with water (2*75 mL).
The organic phase was dried over anhydrous sodium sulphate and concentrated under vacuum.
This crude mass (11.5 gm) was then dissolved in tetrahydrofuran (80 mL).
Sodium acetate (6.67 gm, 83.3 mmol) was added at 0° C. to this solution and stirred at room temperature for 14 hours.
The reaction mixture was then filtered through celite bed and the bed was washed with ethyl acetate (2*50 mL).
The filtrate and the washings were mixed and concentrated under vacuum.
To this crude mass (6.6 g), 5N HCl (50 mL) was added at 0° C. and stirred at room temperature for 5 h.
The reaction mixture was then extracted with ethyl acetate (3*75 mL), the combined organic phase was washed with water (2*25 mL), dried over anhydrous sodium sulphate and concentrated under vacuum to give a crude mass which was purified using silica gel column chromatography to give the desired product (4.64 g 73% yield).
1H NMR (CDCl3): 6.04 (d, 1H), 5.27 (br s, 1H), 2.11 (s, 3H).
References:
US2016/66574,2016,A1 Location in patent:Paragraph 0229; 0230; 0231