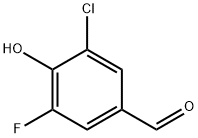
3-CHLORO-5-FLUORO-4-HYDROXYBENZALDEHYDE synthesis
- Product Name:3-CHLORO-5-FLUORO-4-HYDROXYBENZALDEHYDE
- CAS Number:870704-13-5
- Molecular formula:C7H4ClFO2
- Molecular Weight:174.56
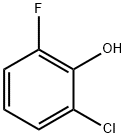
2040-90-6
238 suppliers
$5.00/1g
100-97-0
0 suppliers
$14.00/250G

870704-13-5
32 suppliers
$32.00/1g
Yield:870704-13-5 40%
Reaction Conditions:
with trifluoroacetic acid at 60; for 16 h;Inert atmosphere;
Steps:
9 3-chloro-5-fhioro-4-hvdroxybenzaldehyde
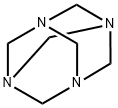
2-fluoro-6-chlorophenol (0.5 mg, 3.5mmol) and hexamethylenetetramine (0.5 g, 3.5 mmol) were dissolved in TFA (3 mL) and stirred at 60 °C for 16 h. The mixture was poured into ice -water (20 mL) and extracted with Et20 (3 x 20 mL). The organic fractions were combined, washed with brine (25 mL), dried over MgSCf, filtered, and the solvent was evaporated. The product, purified by column chromatography (hexane :EtO Ac; 7:3), was obtained as a white solid (250 mg, 40 %). (0157) NMR (500 MHz, CDCfs) (ppm) 9.83 (d, / = 2.1 Hz, 1H), 7.74 - 7.71 (m, 1H), 7.59 (dd, / = 9.6, 1.8 Hz, 1H), 6.12 (s, 1H); (0158) 13C NMR (126 MHz,CDCf) (ppm) 188.8, 151.6 (d, / = 248.2 Hz), 146.2 (d, / = 15.3 Hz), 129.7 (d, / = 5.4 Hz), 127.5 (d, / = 3.1 Hz), 122.7, 115.4 (d, / = 18.5 Hz); 19F NMR (471 MHz, CDCl3) (0159) HRMS calcd for C7H3CIFO2 [ M-I I f 172.9811, found 172.9809.
References:
WO2019/201867,2019,A1 Location in patent:Page/Page column 11; 20; 21