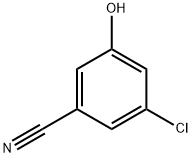
3-chloro-5-hydroxy-benzonitrile synthesis
- Product Name:3-chloro-5-hydroxy-benzonitrile
- CAS Number:473923-97-6
- Molecular formula:C7H4ClNO
- Molecular Weight:153.57
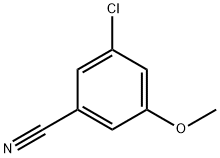
473923-96-5

473923-97-6
General procedure for the synthesis of 3-chloro-5-hydroxybenzonitrile from 3-chloro-5-methoxybenzonitrile: 3-chloro-5-methoxybenzonitrile (7.0 g, 41.766 mmol) and 2,4,6-trimethylpyridine (100 mL) were added to a 250 mL three-necked flask. The reaction mixture was heated to 170 °C, followed by the addition of lithium iodide (16.76 g, 125.298 mmol) and the reaction was continued with stirring for 4 h at this temperature. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the complete consumption of raw materials, the reaction mixture was cooled to room temperature. The reaction was quenched with 10% aqueous hydrochloric acid and the reaction mixture was subsequently extracted with ethyl acetate (EtOAc). The organic phase was washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate (Na2SO4) and filtered. The solvent was removed by concentration under reduced pressure to give a yellow oily crude product. Purification by silica gel column chromatography using ethyl acetate/hexane (10:90, v/v) as eluent gave 3-chloro-5-hydroxybenzonitrile (6.0 g, 94% yield) as a white solid.

473923-96-5
65 suppliers
inquiry

473923-97-6
186 suppliers
$9.00/1g
Yield:473923-97-6 94%
Reaction Conditions:
Stage #1:3-chloro-5-methoxybenzonitrile with lithium iodide in 2,4,6-trimethyl-pyridine at 170; for 4 h;
Stage #2: with hydrogenchloride in 2,4,6-trimethyl-pyridine;water at 20;
Steps:
3.2; 3.3
A 250 mL flask was charged with 24b (7.0 g, 41.766 mmol) and 2,4,6-collidine (100 mL). The mixture was heated to 170° C. and Lil (16.76 g, 125.298 mmol) was added and the reaction mixture was heated for 4 h. When 24b was consumed the reaction was cooled to RT and quenched with 10% aqueous HCl. The resulting mixture was extracted with EtOAc and washed with water and brine. The EtOAc extract was dried(Na2SO4) and filtered. The solvent was removed in vacuo to afford a yellow oil which was purified by silica gel chromatography eluting with EtOAc/hexane (10:90) to afford 6.0 g (94%) of 24c.
References:
Roche Palo Alto LLC US2007/88015, 2007, A1 Location in patent:Page/Page column 22
6575-00-4
272 suppliers
$6.00/1g

473923-97-6
186 suppliers
$9.00/1g
![3-Chloro-5-[(4-Methoxybenzyl)oxy]benzonitrile](/CAS/20150408/GIF/1213790-87-4.gif)
1213790-87-4
19 suppliers
inquiry

473923-97-6
186 suppliers
$9.00/1g
766-84-7
272 suppliers
$9.00/10g

473923-97-6
186 suppliers
$9.00/1g