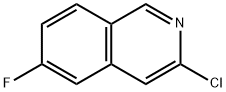
3-Chloro-6-fluoro-isoquinoline synthesis
- Product Name:3-Chloro-6-fluoro-isoquinoline
- CAS Number:1041423-28-2
- Molecular formula:C9H5ClFN
- Molecular Weight:181.59
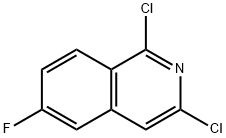
1041423-26-0

1041423-28-2
General procedure for the synthesis of 3-chloro-6-fluoroisoquinoline using 1,3-dichloro-6-fluoroisoquinoline as starting material: hydriodic acid (20 mL, 45% aqueous solution) and red phosphorus (0.9 g, 28.9 mmol) were added to a stirring solution of 1,3-dichloro-6-fluoroisoquinoline (2.5 g, 11.6 mmol) in acetic acid (40 mL) at room temperature. The reaction mixture was stirred at 100 °C for 4 hours. After the reaction was completed, it was cooled to room temperature and the mixture was concentrated under reduced pressure. The residue was dissolved in dichloromethane (100 mL) and washed with saturated aqueous sodium bicarbonate (2 x 100 mL). The organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure to give 3-chloro-6-fluoroisoquinoline as a dark gray solid. Mass spectrum (ESI, m/z): 182.0 [M + 1]+; 1H NMR (400 MHz, DMSO-d6) δ 9.24 (s, 1H), 8.32-8.29 (m, 1H), 8.04 (s, 1H), 7.82-7.76 (m, 1H), 7.66-7.61 (m, 1H).

1041423-26-0
60 suppliers
$50.00/100mg

1041423-28-2
47 suppliers
$71.00/100mg
Yield:1041423-28-2 77%
Reaction Conditions:
with phosphorus;hydrogen iodide in water;acetic acid at 100; for 4 h;
Steps:
9.3 Synthesis of 3-chloro-6-fluoroisoquinoline (9-3).
To a stirred solution of 1,3 -dichloro-6- fluoroisoquinoline (2.5 g, 1 1.6 mmol) in acetic acid (40 mL) and hydriodic acid (20 mL, 45% aqueous solution) was added red phosphorus (0.9 g, 28.9 mmol) at ambient temperature. The resulting mixture was stirred for 4 hours at 100 °C. After cooling down to ambient temperature, the resulting mixture was concentrated under reduced pressure. The residue was dissolved into dichloromethane (100 mL) and washed with saturated aqueous solution of sodium bicarbonate (2 x 100 mL). The organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure to afford 3-chloro-6-fluoroisoquinoline as a dark grey solid: MS (ESI, m/z): 182.0 [M + 1]+; 1H MR (400 MHz, OMSO-d6) δ 9.24 (s, 1H), 8.32-8.29 (m, 1H), 8.04 (s, 1H), 7.82-7.76 (m, 1H), 7.66-7.61 (m, 1H).
References:
MERCK SHARP & DOHME CORP.;ABBAS, Walji;HOSTETLER, Eric;GRESHOCK, Thomas;LI, Jing;MOORE, Keith P.;BENNACEF, Idriss;MULHEARN, James;SELNICK, Harold;WANG, Yaode;YANG, Kun;FU, Jianmin WO2015/188368, 2015, A1 Location in patent:Page/Page column 52-53
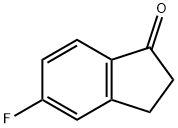
700-84-5
292 suppliers
$7.00/1g

1041423-28-2
47 suppliers
$71.00/100mg