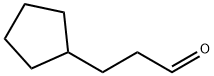
3-cyclopentylpropanal synthesis
- Product Name:3-cyclopentylpropanal
- CAS Number:6053-89-0
- Molecular formula:C8H14O
- Molecular Weight:126.2
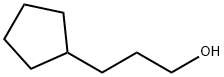
767-05-5
63 suppliers
$20.00/100mg

6053-89-0
28 suppliers
$45.00/50mg
Yield:6053-89-0 83%
Reaction Conditions:
Stage #1: 3-cyclopentyl-1-propanolwith oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 0.5 h;
Stage #2: with triethylamine in dichloromethane at -78 - 20;
Steps:
136.136A
Example 136; N-r(5Z)-2-tert-butyl-4-(cvclopentylmethyl)isothiazol-5(2H)-ylidene]-5-chloro-2- methoxybenzamide; Example 136A; 3 -cyclopentylpropanal; To CH2Cl2 (100 mL) at -78 0C were added oxalyl chloride (4.1 mL, 46.8 mmol, Aldrich) and dry DMSO (5.5 mL, 78.0 mmol, Aldrich), dropwise. After 5 min, 3- cyclopentylpropan-1-ol (5.0 g, 39.0 mmol, Aldrich) in 5 mL Of CH2Cl2 was added. The mixture was stirred for an additional 0.5 hr at -78°C, and triethylamine (27.2 mL, 195.0 mmol) was added. The mixture was then allowed to warm to room temperature over 0.5 hr. After stirring for 3 hr, 100 mL of water was added. The phases were separated, and the aqueous phase was extracted with diethyl ether (3 x 100 mL). The combined organic extracts were washed successively with 50 rnL of aqueous 1% HCl, 50 rnL of water, 50 mL of aqueous 5% NaHCO3 and 50 mL of saturated aqueous NaCl. The organic layer was dried (MgSO4), filtered and concentrated to provide 4.1 g (83%) of the title compound. MS (ESI+) m/z 144 (M+NH4)+.
References:
WO2010/54024,2010,A2 Location in patent:Page/Page column 126-127
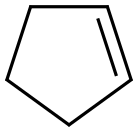
142-29-0
263 suppliers
$14.00/25mL
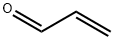
107-02-8
0 suppliers
$38.60/4s8501

6053-89-0
28 suppliers
$45.00/50mg