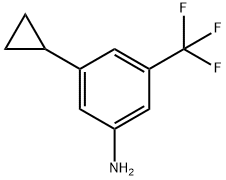
3-CYCLOPROPYL-5-(TRIFLUOROMETHYL)ANILINE synthesis
- Product Name:3-CYCLOPROPYL-5-(TRIFLUOROMETHYL)ANILINE
- CAS Number:1057079-57-8
- Molecular formula:C10H10F3N
- Molecular Weight:201.19
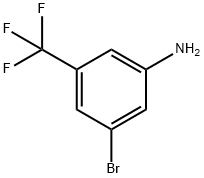
54962-75-3
307 suppliers
$5.00/1g
411235-57-9
427 suppliers
$6.00/1g

1057079-57-8
8 suppliers
inquiry
Yield:1057079-57-8 55%
Reaction Conditions:
palladium diacetate;tricyclohexylphosphine in water;toluene at 100; for 2 h;Inert atmosphere;
Steps:
30.a
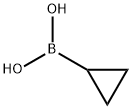
Example 30: Synthesis of c/s-N-(3-cyclopropyl-5-(trifluoromethyl)phenyl)-3-ethyl-4- methyl-l-(2,6-dimethyIphenyl)-5-oxopyrrolidine-3-carboxamide[0167] a) A 40 mL vial was charged with 3-amino-5-bromobenzotrifluoride (275 mg, 1.15 mmol), cyclopropylboronic acid (128 mg, 1.5 mmol), Pd(OAc)2 (13 mg, 0.06 mmol), triclyclohexylphosphine (38 mg, 0.13 mmol), and K3P04 (488 mg, 2.3 mmol). The vial was subsequently sealed with a septum-lined screw cap and purged with nitrogen, followed by the addition of degassed toluene (4 mL) and degassed water (1 mL). After stirring the reaction mixture for 2 h at 100 °C, the mixture was diluted in ethyl acetate (50 mL) and the organics were washed with brine, dried (MgS04), and concentrated in vacuo. The residue was purified by flash chromatography (Si02, 10-40% EtOAc/hexanes) to give the desired product in 55% yield (126 mg). LC-MS Rt (retention time): 2.46 min, MS: (ES) m/z 202 (M+H+). [0168]
References:
WO2011/35332,2011,A1 Location in patent:Page/Page column 69