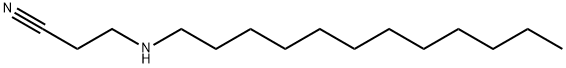
3-(DODECYLAMINO)PROPIONITRILE synthesis
- Product Name:3-(DODECYLAMINO)PROPIONITRILE
- CAS Number:4763-40-0
- Molecular formula:C15H30N2
- Molecular Weight:238.41

124-22-1
412 suppliers
$5.00/25g
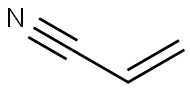
107-13-1
431 suppliers
$19.00/25ML

4763-40-0
28 suppliers
$19.00/1g
Yield:4763-40-0 98%
Reaction Conditions:
in neat (no solvent, gas phase) at 40; for 2 h;Microwave irradiation;Molecular sieve;Green chemistry;Michael Addition;
Steps:
2.6 n-dodecyliminopropionitrile (4d, C15H30N2)
To 229.97μL n-dodecylamine (1mmol) dissolved in 164.57μL (2.5mmol) acrylonitrile 0.12g molecular sieve were added. The reaction mixture was mixed properly with the vortex mixture and heated at 40°C under microwave irradiation for 2h, cooled, and centrifuged. The reaction was monitored by TLC (30% ethyl acetate as eluent). After completion of reaction, it was cooled down to room temperature and catalyst was separated by centrifuge technique at 2500r.p.m. for 5min. The supernatant was collected and further purified by column chromatography. Upon isolation of product the solvents were evaporated. Recrystallization from methanol afforded 233.24g (98%) 4d. B.p.: 530-532°C; Rf=0.75; 1H NMR (300MHz, CDCl3): δ=0.88 (t, 3H, J=6.9Hz, CH3 terminal), 1.26 {m, 2H, CH2 (dodecyl)}, 2 (m, 1H, NH), 2.53 {t, 2H, J=6.6Hz, CH2-NH (dodecyl)}, 2.8 {t, 2H, J=6.6Hz, CH2-NH (propyl)}, 2.9 (t, 2H, J=6.6Hz, CH2-CN) ppm; 13C{1H} NMR (300MHz, CDCl3): δ=14.1 (-CH3), 31.9 (-CH2-), 118 (C≡N). IR: νmax=2251 (C≡N stretching), 2853-2920 (C-H stretching), 1129 (C-N stretch) cm-1; MS: m/z=238 (M+), calcd for C9H15N3 238, found=239.
References:
Das, Parineeta;Devi, Nirmala;Puzari, Amrit [Journal of the Indian Chemical Society,2022,vol. 99,# 5,art. no. 100411]

124-22-1
412 suppliers
$5.00/25g

107-13-1
431 suppliers
$19.00/25ML
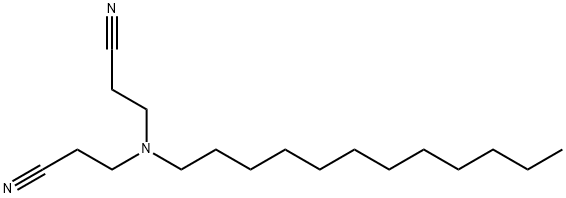
1555-62-0
12 suppliers
inquiry

4763-40-0
28 suppliers
$19.00/1g