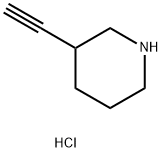
3-Ethynylpiperidine hydrochloride synthesis
- Product Name:3-Ethynylpiperidine hydrochloride
- CAS Number:959918-19-5
- Molecular formula:C7H12ClN
- Molecular Weight:145.63
664362-16-7

959918-19-5
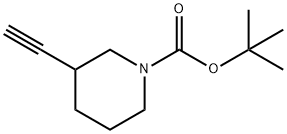
General procedure for the synthesis of 3-ethynylpiperidine hydrochloride from 3-ethynylpiperidine-1-carboxylic acid tert-butyl ester: Preparation of Intermediate 35 (Method V); Synthesis of 3-ethynylpiperidine hydrochloride: to Intermediate 33 (1.50 g, 7.18 mmol) was added a dioxane solution (50 mL) of 4 M HCl. The reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the mixture was concentrated in vacuum. The residue was ground with ether (Et2O) to give 3-alkynylpiperidine hydrochloride (0.91 g, 86% yield).1H NMR (DMSO-d6) δ: 9.02 (2H, br.s), 3.38-3.24 (1H, m), 3.21-3.08 (2H, m), 2.94-2.76 (3H, m), 2.00-1.87 (1H, m), 1.83-1.73 (1H, m), 1.73-1.60 (1H, m), 1.60-1.48 (1H, m).

664362-16-7
119 suppliers
$43.00/100mg

959918-19-5
67 suppliers
$110.00/50mg
Yield: 86%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 16 h;
Steps:
INTERMEDIATE 35 (METHOD V); 3-Ethynylpiperidine hydrochloride; To Intermediate 33 (1.50 g, 7.18 mmol) was added HCl (4iVm dioxane, 50 mL). The reaction mixture was stirred at r.t. for 16 h and concentrated in vacuo. Trituration with Et2O gave the title compound (0.91 g, 86%). δH (DMSOd6) 9.02 (2H, br. s), 3.38- 3.24 (IH, m), 3.21-3.08 (2H, m), 2.94-2.76 (3H, m), 2.00-1.87 (IH, m), 1.83-1.73 (IH, m), 1.73-1.60 (IH, m), 1.60-1.48 (IH, m).
References:
UCB PHARMA S.A. WO2007/141504, 2007, A1 Location in patent:Page/Page column 59