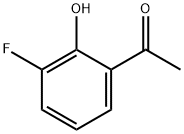
3''-Fluoro-2''-Hydroxyacetophenone synthesis
- Product Name:3''-Fluoro-2''-Hydroxyacetophenone
- CAS Number:699-92-3
- Molecular formula:C8H7FO2
- Molecular Weight:154.14
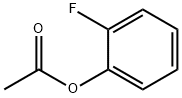
29650-44-0
40 suppliers
$70.00/1g
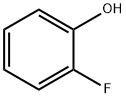
367-12-4
421 suppliers
$5.00/10g
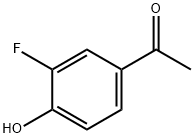
403-14-5
189 suppliers
$16.00/1 g

699-92-3
94 suppliers
$54.00/250mg
Yield: 58% , 24% , 70 %Chromat.
Reaction Conditions:
with aluminum (III) chloride in chlorobenzene at 120;Inert atmosphere;Large scale;Fries Phenol Ester Rearrangement;Solvent;Temperature;
Steps:
Representative procedure for Fries rearrangement
A solution of 2-fluorophenyl acetate (3, 1.070 Kg, 6.94 moles) and monochlorobenzene (5.34 Lit.) was heated to 120oC. Aluminum trichloride (1.39 Kg, 10.42 moles) was added lot wise at 120 °C over 70-75min. The rise in temperature (~5oC) was controlled by rate of addition. The reaction was maintained at the same temperature for additional 3 h. The reaction mass was cooled to 0 - 5oC. Water (1.07 Lit.) followed by 3.5% aq. HCl (2.14 Lit.) was added gradually by maintaining internal temperature <30oC using ice bath with vigorous stirring. The mixture was stirred vigorously at 5-10oC for 1 h and filtered, washed the solid cake with water (1 Lit.), suck dried the cake to get beige solid which gave para-isomer 4 (416 g, 38.8%, >98% HPLC purity). The organic layers was separated from the filtrate and washed with water (2 Lit.). Concentration of organic layer at normal pressure provided the crude product (530 g) which was then purified by steam distillation. The first fraction was a liquid enriched with 2-fluorophenol (45.2 g, LC: 70 % 2-fluorophenol, 4 % 4, 21 % 5) and second fraction of pale yellow solid as ortho-isomer 5. The product 5 was then isolated by filtration, washed with water (80 mL) and dried under vacuum to yield the title compound (257.6 g, 24%, > 98.2 % HPLC) as a pale yellow solid. Steam distillation residue (HPLC: 92.7 % 4,3.63 % 5) crystallized from MTBE:Hexane to furnish compound 4 (205.4g, 19.2%, >98% HPLC purity). 3-fluoro-4-hydroxyacetophenone (4).1H NMR (CDCl3, 400 MHz) δ: 7.75-7.67 (m, 2H, ArH), 7.06 (t, 1H, J = 8.4 Hz, ArH), 5.98 (d,1H, J = 4 Hz, Ar-OH), 2.56 (s, 3H, Ac). Observed LCMS 153.00. 3-fluoro-2-hydroacetophenone (5).1HNMR (CDCl3, 400 MHz) δ:12.29 (s, 1H, OH), 7.56-7.51 (m, 1H, ArH), 7.32-7.25 (m, 1H, ArH), 6.88-6.81 (m, 1H, ArH), 2.66 (s, 3H, CH3). Observed LCMS 153.00.
References:
Yerande, Swapnil G.;Shendage, Deepak M.;Wakchaure, Prasad B.;Phadtare, Ganesh R.;Bhoite, Madhavrao Y.;Gangopadhyay, Ashok Kumar;Nagarajan, Kuppuswamy;Rupp, Richard Helmut [Tetrahedron Letters,2014,vol. 55,# 15,p. 2426 - 2429] Location in patent:supporting information

29650-44-0
40 suppliers
$70.00/1g

699-92-3
94 suppliers
$54.00/250mg

367-12-4
421 suppliers
$5.00/10g

75-36-5
579 suppliers
$17.92/100G

699-92-3
94 suppliers
$54.00/250mg

29650-44-0
40 suppliers
$70.00/1g

403-14-5
189 suppliers
$16.00/1 g

699-92-3
94 suppliers
$54.00/250mg

367-12-4
421 suppliers
$5.00/10g

699-92-3
94 suppliers
$54.00/250mg