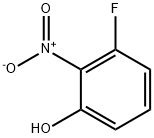
3-Fluoro-2-nitrophenol synthesis
- Product Name:3-Fluoro-2-nitrophenol
- CAS Number:385-01-3
- Molecular formula:C6H4FNO3
- Molecular Weight:157.1
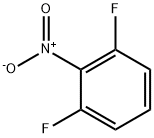
19064-24-5

385-01-3
General procedure for the synthesis of 3-fluoro-2-nitrophenol from 2,6-difluoronitrobenzene: First, potassium TERT-butoxide (1.23 g, 11 mmol) was dissolved in 25 mL of anhydrous DMSO and stirred for 30 min at room temperature. Subsequently, 1,3-difluoro-2-nitrobenzene (1.59 g, 10 mmol) was added and the reaction was continued with stirring for 18 hours. After completion of the reaction, the mixture was diluted with 150 mL of aqueous 1N sulfuric acid and extracted with three 50 mL portions of diethyl ether. The organic layers were combined, washed with water, dried over sodium sulfate, filtered and concentrated in vacuum. The residue was dissolved in 50mL of trifluoroacetic acid and reacted for 30 minutes at room temperature before being concentrated again under vacuum. Next, the residue was treated with 50mL of 1N aqueous sodium hydroxide and extracted with three 30mL portions of diethyl ether. The aqueous layer was acidified with 1N aqueous sulfuric acid solution and extracted with two 50mL dichloromethane. The dichloromethane layers were combined, washed with water, dried over sodium sulfate, filtered and concentrated in vacuum to give 1.3 g of 3-fluoro-2-nitrophenol as an orange oil (61% yield). Next, 3-fluoro-2-nitrophenol (1.13 g, 7.2 mmol) and pyridine (0.65 mL, 8 mmol) were dissolved in 15 mL of anhydrous dichloromethane and cooled in an ice bath. A solution of trifluoromethanesulfonic anhydride (1.33 mL, 7.9 mmol) dissolved in 3 mL of anhydrous dichloromethane was added slowly. After 4 hours of reaction, the reaction mixture was diluted with 100 mL of dichloromethane and washed sequentially with two 30 mL aqueous saturated sodium bicarbonate solutions, two 30 mL aqueous 1N sulfuric acid solutions, and two 30 mL of water. The organic layer was dried over sodium sulfate, filtered and concentrated in vacuum to give 2 g of the target product as a light brown oil (96% yield).

19064-24-5
278 suppliers
$6.00/1g

385-01-3
176 suppliers
$5.00/50mg
Yield: 61%
Reaction Conditions:
with potassium tert-butylate in dimethyl sulfoxide at 20; for 18.5 h;
Steps:
26
A solution of potassium TERT-BUTOXIDE (1.23 g, 11 mmol) in 25 mL of anhydrous DMSO was stirred at room temperature for 30 minutes and treated with 1, 3-difluoro-2-nitrobenzene (1.59 g, 10 mmol). After 18 hours, the mixture was diluted with 150 mL of 1 N aqueous sulfuric acid and extracted with three 50 mL portions of diethyl ether. The combined organic layers were washed with water, dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was dissolved in 50 mL of trifluoroacetic acid. After 30 minutes at room temperature, the mixture was concentrated in vacuo, treated with 50 mL of 1 N aqueous sodium hydroxide, and extracted with three 30 mL portions of diethyl ether. The aqueous layer was acidified with 1 N aqueous sulfuric acid and extracted with two 50 mL portions of dichloromethane. The combined dichloromethane layers were washed with water, dried over sodium sulfate, filtered, and concentrated in vacuo to give 1.3 g of 3-fluoro-2-nitrophenol as orange oil (61% yield). An solution of 3-fluoro-2-nitrophenol (1.13 g, 7.2 mmol) and pyridine (0.65 mL, 8 mmol) in 15 mL of anhydrous dichloromethane was cooled in an ice bath and treated with a solution of triflic anhydride (1.33 mL, 7.9 mmol) in 3 mL of anhydrous dichloromethane. After 4 hours, the reaction mixture was diluted with 100 mL of dichloromethane, washed with two 30 mL portions of saturated aqueous sodium bicarbonate, two 30 mL portions of 1 N aqueous sulfuric acid, and two 30 mL portions of water, dried over sodium sulfate, filtered, and concentrated in VACUA TAO give 2 g of the title product as a light brown oil (96% yield).
References:
BOEHRINGER INGELHEIM PHARMACEUTICALS, INC. WO2005/30213, 2005, A1 Location in patent:Page/Page column 182-183
641-49-6
110 suppliers
inquiry

385-01-3
176 suppliers
$5.00/50mg
372-20-3
391 suppliers
$7.00/5g

385-01-3
176 suppliers
$5.00/50mg

372-20-3
391 suppliers
$7.00/5g
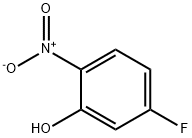
446-36-6
270 suppliers
$9.00/10g

385-01-3
176 suppliers
$5.00/50mg
394-41-2
299 suppliers
$16.00/1g

372-20-3
391 suppliers
$7.00/5g
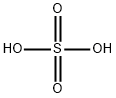
7664-93-9
0 suppliers
$21.64/1003

385-01-3
176 suppliers
$5.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
361-48-8
0 suppliers
inquiry