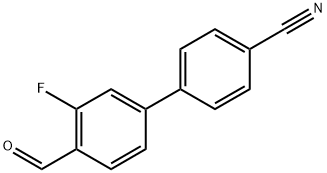
3'-Fluoro-4'-formyl-[1,1'-biphenyl]-4-carbonitrile synthesis
- Product Name:3'-Fluoro-4'-formyl-[1,1'-biphenyl]-4-carbonitrile
- CAS Number:869767-75-9
- Molecular formula:C14H8FNO
- Molecular Weight:225.22
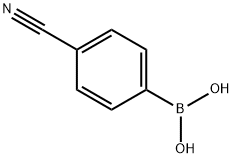
126747-14-6
408 suppliers
$6.00/1g
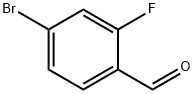
57848-46-1
383 suppliers
$9.00/1g
![3'-Fluoro-4'-formyl-[1,1'-biphenyl]-4-carbonitrile](/CAS/20211123/GIF/869767-75-9.gif)
869767-75-9
2 suppliers
inquiry
Yield:869767-75-9 94%
Reaction Conditions:
with potassium carbonate;palladium dichloride in water at 20; for 3 h;Inert atmosphere;Suzuki-Miyaura Coupling;
Steps:
4.2.2. Preparation of compounds 9b-c and 9e-f
General procedure: Different substituted 4-bromobenzylaldehyde (1.0 mmol, 1.0equiv.) was dissolved in PEG400/H2O (6 mL/6 mL) in the present of(4-cyanophenyl)boronic acid 7 (1.2 mmol, 1.2 equiv.), PdCl2(0.005 mmol, 0.005 equiv.) and potassium carbonate (3.0 mmol, 3.0equiv.). The reaction mixture was stirred at room temperature for3 h until complete consumption of starting material as judged byTLC, then poured into water (20 mL) and extracted with ethyl acetate(10 mLx3). The organic layers were washed with brine, driedover anhydrous Na2SO4, filtered and concentrated under vacuum togive the crude product that was purified by column chromatography on silica gel, eluting with EtOAc/petroleum ether to give the products 9b-c and 9e-f.
References:
Chen, Xiaomei;Ding, Li;Tao, Yuan;Pannecouque, Christophe;De Clercq, Erik;Zhuang, Chunlin;Chen, Fen-Er [European Journal of Medicinal Chemistry,2020,vol. 202,art. no. 112549]