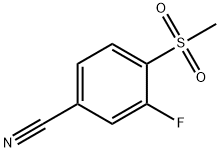
3-Fluoro-4-(methylsulphonyl)benzonitrile synthesis
- Product Name:3-Fluoro-4-(methylsulphonyl)benzonitrile
- CAS Number:185946-05-8
- Molecular formula:C8H6FNO2S
- Molecular Weight:199.2
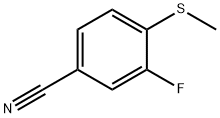
185946-04-7
3 suppliers
inquiry

185946-05-8
18 suppliers
inquiry
Yield:185946-05-8 95%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 20; for 19.5 h;Cooling with ice;
Steps:
2 3-Fluoro-4-methylsulfonylbenzonitrile
Reference Example 2 3-Fluoro-4-methylsulfonylbenzonitrile 3-Chloroperbenzoic acid (24.3 g, 91.7 mmol) was added to a methylene chloride (220 mL) solution of the compound obtained in Reference Example 1 (7.30 g, 43.7 mmol) under ice water cooling, and the mixture was stirred for 30 minutes at the same temperature and then was further stirred for 19 hours at room temperature. A saturated aqueous solution of sodium hydrogen carbonate was added to the reaction mixture, and the mixture was subjected to extraction two times with methylene chloride. The organic layer thus obtained was washed with a 1.5M aqueous solution of sodium sulfite, and then was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure. The resulting residue was washed with hexane-ethyl acetate (5:1, v/v). Thus, the title compound (8.27 g, yield: 95%) was obtained. 1H-NMR (400 MHz, CDCl3) δppm: 8.12 (1H, dd, J=9 Hz, 8 Hz), 7.67 (1H, dd, J=8 Hz, 1 Hz), 7.58 (1H, dd, J=9 Hz, 1 Hz), 3.27 (3H, s).
References:
US2012/129891,2012,A1 Location in patent:Page/Page column 7
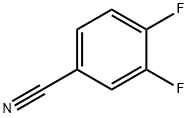
64248-62-0
448 suppliers
$6.00/5g

185946-05-8
18 suppliers
inquiry