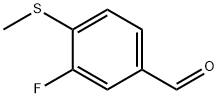
3-Fluoro-4-(methylthio)benzaldehyde synthesis
- Product Name:3-Fluoro-4-(methylthio)benzaldehyde
- CAS Number:177756-61-5
- Molecular formula:C8H7FOS
- Molecular Weight:170.2
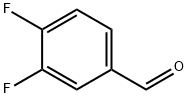
34036-07-2
367 suppliers
$7.00/5g
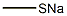
5188-07-8
298 suppliers
$13.00/5g

177756-61-5
11 suppliers
inquiry
Yield:-
Reaction Conditions:
in acetonitrile; for 19 h;
Steps:
Preparation 49: 3-Fluoro-4-methanesulfinylbenzoic acid and 3-fluoro-4-methylsulfonylbenzoic acid : A stirred solution of 3,4-difluorobenzaldehyde (5.0 g, 35.2 mmol) in dry acetonitrile (50 mL) under argon was treated portionwise with with sodium thiomethoxide (2.47 g, 35.2 mmol) over approximately 1 h. After 18 h the reaction mixture was diluted with EtOAc (50 mL) and washed with saturated aqueous NaHCO3 (2 x 10 mL) and saturated aqueous NH4Cl (20 mL). The organic phase was dried (MgSO4), evaporated and the residue purified by column chromatography (IH-EtOAc 9:1 then 7:3) to give 3-fluoro-4-methylsulfanylbenzaldehyde: δH (CDCl3) 2.54 (3H, s), 7.32 (IH, t), 7.51 (IH, dd), 7.63 (IH, dd), 9.92 (IH, d). A sample of this thioether (1.0 g, 5.38 mmol) was suspended in water and NaH2PO4 (705 mg, 5.88 mmol), tert- butanol (44 mL) and sodium chlorite (1.59 g, 63 mmol) were then added. After stirring vigorously for 1.5 h, the tert-butanol was removed under reduced pressure and EtOAc (50 mL) added. The mixture was extracted with 1 M aqueous NaOH (3 x 20 mL) and the combined extracts acidified to pH 2 using dilute HCl. The precipitate was extracted into EtOAc which was dried (MgSO4) and evaporated to afford an inseparable mixture of the the title sulfoxide: RT = 2.22 min (method 2); m/z (ES+) = 203.0 [M+H]+ and the sulfone 2.22 min (method 2); m/z (ES+) = 219.0 [M+H]+.
References:
WO2007/3960,2007,A1 Location in patent:Page/Page column 33-34