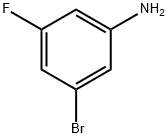
3-Fluoro-5-bromoaniline synthesis
- Product Name:3-Fluoro-5-bromoaniline
- CAS Number:134168-97-1
- Molecular formula:C6H5BrFN
- Molecular Weight:190.01
807620-95-7

134168-97-1
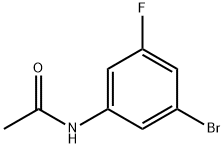
N-(3-bromo-5-fluorophenyl)acetamide (Intermediate 181, 8.7 g, 37.4 mmol) was used as a raw material, which was dissolved in ethanol (30 mL) and then concentrated hydrochloric acid (80 mL) was slowly added. The reaction mixture was heated to 100 °C and kept for 1 h for the reaction. After completion of the reaction, the mixture was cooled to room temperature and neutralized with 5 N sodium hydroxide solution. The crude product was extracted with ethyl acetate (2 x 100 mL) and the combined organic layers were washed with brine and dried over anhydrous magnesium sulfate. After filtration, the solvent was removed by concentration under reduced pressure. Purification by silica gel column chromatography using a gradient elution with 5-10% ethyl acetate in hexane solution gave 5.3 g (75% yield) of the yellow oily target product 3-bromo-5-fluoroaniline (Intermediate 180). Mass spectrum (ES+): m/z 190, 192 ([M+H]+), molecular formula C6H5BrFN. 1H NMR (DMSO-d6) δ: 5.46-6.00 (m, 2H), 6.24-6.37 (m, 1H), 6.44-6.53 (m, 1H), 6.54-6.61 (m, 1H).
7087-65-2
118 suppliers
$14.00/1 g
7439-89-6
467 suppliers
$10.00/25g

134168-97-1
119 suppliers
$13.00/250mg
Yield:-
Reaction Conditions:
with ammonium chloride in water
Steps:
A.14.3 Step 3
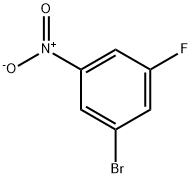
Step 3 A suspension of 29.0 g of iron powder and 20.0 g of ammonium chloride in 50 ml of water was heated to 90° C. with vigorous stirring. To this was added 24.0 g of 1-bromo-3-fluoro-5-nitrobenzene portionwise. The mixture was refluxed for 4 hours, cooled to room temperature and filtered to remove insoluble matter. The insoluble matter and the filtrate were extracted, respectively, with ethyl acetate. Both extracts were combined together and evaporated in vacuo to give 12.1 g of the title compound. Vacuum distillation of a portion thereof gave an analytical sample boiling at 103.0°-105.0° C. /7 mm Hg. NMR (DMSO-d6, δ ppm): 5.73 (2H, bs), 6.2-6.4 (1H, m), 6.4-6.7 (2H, m). Analysis calculated for C6 H5 BrFN; C 37.92; H 2.65; N 7.37; Found: C 37.83; H 2.68; N 7.36. Hydrochloride, m.p. 216.0°-219.0° C. (decomp.) from isopropyl alcohol.
References:
Kanebo, Ltd. US5087640, 1992, A

807620-95-7
4 suppliers
inquiry

134168-97-1
119 suppliers
$13.00/250mg
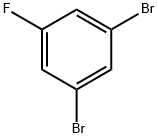
1435-51-4
296 suppliers
$5.00/5g

134168-97-1
119 suppliers
$13.00/250mg

7087-65-2
118 suppliers
$14.00/1 g

134168-97-1
119 suppliers
$13.00/250mg
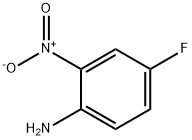
364-78-3
281 suppliers
$7.00/5g

134168-97-1
119 suppliers
$13.00/250mg