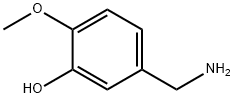
3-Hydroxy-4-methoxy benzylamine synthesis
- Product Name:3-Hydroxy-4-methoxy benzylamine
- CAS Number:89702-89-6
- Molecular formula:C8H11NO2
- Molecular Weight:153.18
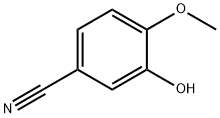
52805-46-6
192 suppliers
$6.00/1g

89702-89-6
23 suppliers
inquiry
Yield:89702-89-6 90%
Reaction Conditions:
with sodium tetrahydroborate;copper ferrite in water; for 0.166667 h;Reflux;Green chemistry;chemoselective reaction;
Steps:
General procedure for the reduction of nitriles to primary amines
General procedure: As a representative example, in a round-bottom flask (15 mL) equipped with a magnetic stirrer, benzonitrile (1 mmol, 0.103 g) was dissolved in H2O (2 mL). Afterward, CuFe2O4 (0.2 mmol, 0.048 g) was added and the mixture was stirred. Then, NaBH4 (2 mmol, 0.076 g) was also added, and the resulting mixture continued to stir at reflux for 5 min. Upon completion of the reaction (monitored by TLC), the mixture was cooled to room temperature, and the catalyst was separated by an external magnet. The reaction mixture was extracted with ethyl acetate (EtOAc) (2 x 4 mL). The organic layers were combined together and dried over anhydrous sodium sulfate (Na2SO4). The solvent was evaporated under reduced pressure. The pure colorless liquid benzylamine was obtained in 95% yield.
References:
Zeynizadeh, Behzad;Mohammad Aminzadeh, Farkhondeh;Mousavi, Hossein [Research on Chemical Intermediates,2019]
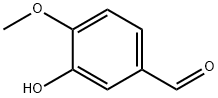
621-59-0
656 suppliers
$9.00/1g

89702-89-6
23 suppliers
inquiry
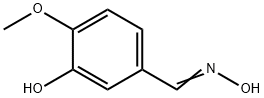
51673-94-0
12 suppliers
inquiry

89702-89-6
23 suppliers
inquiry