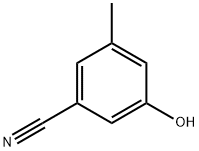
3-hydroxy-5-Methylbenzonitrile synthesis
- Product Name:3-hydroxy-5-Methylbenzonitrile
- CAS Number:95658-81-4
- Molecular formula:C8H7NO
- Molecular Weight:133.15
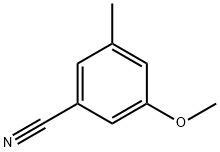
473923-98-7

95658-81-4
In a 100 mL round-bottomed flask, 3-methoxy-5-methylbenzonitrile (500 mg, 3.39 mmol) was dissolved in 15 mL of anhydrous dichloromethane (DCM), followed by the addition of tetrabutylammonium iodide (1.38 g, 3.73 mmol, 3.5 equiv). The reaction system was cooled to -78 °C. Boron tribromide (1 M solution of DCM, 11.87 mL, 33.16 mmol) was added slowly and dropwise at low temperature. The reaction mixture was stirred at -78 °C and then gradually warmed to room temperature and stirred overnight. Upon completion of the reaction, the reaction was quenched with ice water and neutralized with saturated sodium bicarbonate (NaHCO3) solution. The reaction mixture was extracted with dichloromethane (DCM). The organic layers were combined, concentrated and purified by silica gel column chromatography (eluent: 0-10% methanol/DCM) to give a light yellow solid product (408 mg, 90% yield). Liquid chromatography-mass spectrometry (LC-MS) analysis showed a molecular ion peak m/z 132.0 ([M-1]-). Nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 300 MHz, CDCl3) data were as follows: δ 7.20 (s, 1H), 6.96 (s, 1H), 6.91 (s, 1H), 2.37 (s, 3H).

473923-98-7
36 suppliers
inquiry

95658-81-4
61 suppliers
inquiry
Yield:95658-81-4 90%
Reaction Conditions:
with boron trichloride;tetra-(n-butyl)ammonium iodide in dichloromethane at -78 - 20;
Steps:
In a 100 mL round bottom flask, 3- methoxy-5-methyl-benzonitrile (500 mg, 3.39 mmol) was dissolved in 15 mL dry DCM, and tetrabutylammonium iodide (1.38 g, 3.73 mmol, 3.5 eq.) was added. The flask was cooled to -78 0C. BCk (1 M in DCM, 11.87 mL, 33.16 mmol) was added to the reaction dropwise. The reaction mixture was stirred at -78 0C and warmed up to room temperature overnight. The reaction mixture was quenched with ice and neutralized with saturated NaHCCh solution. DCM was added to extract the
References:
WO2009/5674,2009,A2 Location in patent:Page/Page column 423; 424
124289-21-0
114 suppliers
$8.00/100mg

95658-81-4
61 suppliers
inquiry
620-22-4
222 suppliers
$16.00/25mL

95658-81-4
61 suppliers
inquiry

872286-49-2
0 suppliers
inquiry

95658-81-4
61 suppliers
inquiry
585-81-9
86 suppliers
$108.00/5g

95658-81-4
61 suppliers
inquiry