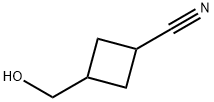
3-(hydroxymethyl)Cyclobutanecarbonitrile synthesis
- Product Name:3-(hydroxymethyl)Cyclobutanecarbonitrile
- CAS Number:938064-72-3
- Molecular formula:C6H9NO
- Molecular Weight:111.14
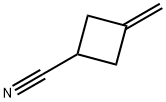
15760-35-7
143 suppliers
$10.00/1g

938064-72-3
10 suppliers
inquiry
Yield:938064-72-3 67%
Reaction Conditions:
Stage #1: 3-methylenecyclobutanecarbonitrilewith 9-bora-bicyclo[3.3.1]nonane in tetrahydrofuran at 0; for 5.25 h;
Stage #2: with sodium perborate;water in tetrahydrofuran; for 12 h;
Steps:
7
Example 7Trans 2-Chloro-iV-[l-cyclopropylmethyl-3-[(propane-l-sulfonylmethyl)- cyclobutylmethyl]-]-4- trifluoromcthyl-bcnzamidc (compound 7):3-Hydroxymcthyl-cyclobutanccarbonitrilc:A solution of 9-BBN in THF (0.5M) (480 mL, 0.24 mol) was added over 1 hour to a stirred solution of 3- methylenecyclobutanecarbonitrile (18.6 g, 0.20 mol) in THF (800 mL) cooled in an ice bath. The reaction mixture was stirred for 0.25 hours then the ice bath removed and stirring continued for a further 4 hours. A suspension of sodium perborate (92.3 g) in water (1000 mL) was added slowly initially and then stirred for 12 hours. The reaction mixture was filtered and the solid washed with diethyl ether (3X). The filtrate was extracted with diethyl ether (3X). The combined organic phase was washed with brine, dried (magnesium sulfate), filtered, and evaporated to leave an oil. The oil was purified using the Horizon Chromatography System (silica column) eluting with 20-100% ethyl acetate-hexane to give a colourless oil (14.9 g, 67%) 1H NMR (500 MHz, CDC13): δ 3.67-3.61 (2H, m), 3.15-3.07 (0.3H, m), 3.06-2.96 (0.7H, m), 2.77-2.69 (0.3H, m), 2.62-2.51 (0.7H, m), 2.51-2.41 (2H, m), 2.60-2.36 (IH, m), 2.31-2.19 (2H, m).
References:
WO2007/60484,2007,A1 Location in patent:Page/Page column 24; 40