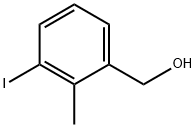
(3-Iodo-2-methylphenyl)methanol synthesis
- Product Name:(3-Iodo-2-methylphenyl)methanol
- CAS Number:76350-89-5
- Molecular formula:C8H9IO
- Molecular Weight:248.06
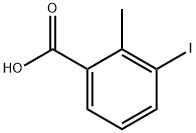
133232-56-1
142 suppliers
$12.00/1g

76350-89-5
37 suppliers
$45.00/50mg
Yield:76350-89-5 92%
Reaction Conditions:
with Trimethyl borate;dimethylsulfide borane complex in tetrahydrofuran at 20; for 1.5 h;Heating / reflux;
Steps:
13; 28.1
To a stirred solution of 3-iodo-2-methylbenzoic acid (40) (5.00 g, 19.1 mmol) and trimethyl borate (7 mL) in THF (5 mL) was added borane-dimethyl sulfide complex (11.5 mL, 2M solution in THF, 23.0 mmol) dropwise at such rate that after gas evolution stopped it remained at a gentle reflux and after the addition was complete the reaction mixture was cooled to room temperature. After 1.5 h the reaction mixture was quenched by slow addition of methanol (10 mL), the resulting mixture was concentrated, and the residue was dissolved in methylene chloride (100 mL). The resulting solution was washed with 2M aqueous sodium hydroxide (80 mL) and water (100 mL), and the organic layer was dried (Na2SO4), filtered and concentrated. The residue was purified by flash column chromatography (silica gel, 60:36:4 methylene chloride/hexanes/MTBE) to give 41 as a white solid. Yield (4.36 g, 92%): 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 7.9 Hz, 1H), 6.89 (t, J= 7.9 Hz, 1H), 4.72 (d, J= 5.8 Hz, 2H), 2.47 (s, 3H), 1.58 (t, J= 5.8 Hz, 1H).
References:
WO2008/131368,2008,A2 Location in patent:Page/Page column 143-144
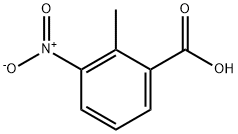
1975-50-4
467 suppliers
$20.00/25g

76350-89-5
37 suppliers
$45.00/50mg