
3-IODOCINNAMIC ACID synthesis
- Product Name:3-IODOCINNAMIC ACID
- CAS Number:41070-12-6
- Molecular formula:C9H7IO2
- Molecular Weight:274.06
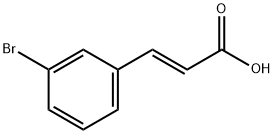
14473-91-7

41070-12-6
General procedure for the synthesis of 3-(3-iodophenyl)acrylic acid from (E)-3-(3-bromophenyl)acrylic acid: CuI (9.6 mg, 0.050 mmol, 5.0 mol%), (E)-3-(3-bromophenyl)acrylic acid (228 mg, 1.00 mmol), NaI (300 mg, 2.00 mmol), evacuated and replaced three times with argon. A dioxane solution (1.0 mL) of racemic trans-N,N'-dimethyl-1,2-cyclohexanediamine (16 μL, 0.10 mmol, 10 mol%) and 1,1,1,3,3,3-hexamethyldisilazane (211 μL, 1.00 mmol) was added under argon protection. The Schlenk tube was sealed with a PTFE valve and the reaction mixture was stirred at 110 °C for 22 hours. Upon completion of the reaction, the suspension was cooled to room temperature, poured into ether (20 mL) and washed with a 10% aqueous hydrochloric acid solution of Na2S2O5 (3 x 20 mL). The organic phase was dried with anhydrous Na2SO4 and concentrated under reduced pressure. The residue was dissolved in hot ethanol (5 mL), hot water (5 mL) was added, and crystallized at 0°C for 15 h. 3-(3-Iodophenyl)acrylic acid (256 mg, 93% yield) was obtained as light yellow needle-like crystals. Melting point: 186-188 °C (literature values 180-182 °C, see Yuzikhin, O. S.; Vasil'ev, A. V.; Rudenko, A. P. Russ. J. Org. Chem. 2000, 36, 1743).1H NMR (400 MHz, DMSO-d6): δ 12.47 (s, 1H) ): δ 12.47 (s, 1H), 8.08 (s, 1H), 7.77 (d, J = 7.8 Hz, 1H), 7.72 (d, J = 7.8 Hz, 1H), 7.52 (d, J = 16.0 Hz, 1H), 7.22 (t, J = 7.8 Hz, 1H), 6.58 (d, J = 16.0 Hz, 1H).13C NMR (100 MHz, DMSO-d6 ): δ 167.3, 142.3, 138.6, 136.6, 136.5, 130.9, 127.4, 120.6, 95.4.

14473-91-7
81 suppliers
$14.00/1g

41070-12-6
52 suppliers
$182.00/250mg
Yield:41070-12-6 93%
Reaction Conditions:
with trans-N,N'-dimethylcyclohexane-1,2-diamine;1,1,1,3,3,3-hexamethyl-disilazane;sodium iodide;copper(l) iodide in 1,4-dioxane at 110; for 22 h;
Steps:
74 Example [74]; Preparation of 3-iodocinnamic acid through an in situ generated [TRIMETHVLSILYL] ester
A Schlenk tube was charged with Cul (9.6 mg, 0.050 mmol, 5.0 mol%), 3- bromocinnamic acid (228 mg, 1.00 mmol), NaI (300 mg, 2.00 mmol), evacuated and backfilled with argon. Racemic trans-N,N'-dimethyl-1, 2-cyclohexanediamine (16 μL, 0.10 mmol, 10 mol%), 1,1, 1,3, 3, [3-HEXAMETHYLDISILAZANE] (211 [I1L,] 1.00 mmol), and dioxane (1.0 mL) were added under argon. The Schlenk tube was sealed with a Teflon valve and the reaction mixture was stirred at [110 °C] for 22 h. The resulting suspension was allowed to reach room temperature, poured into ether (20 mL), and washed with a solution of Na2S205 (100 mg) in 10% aq [HC1] [(3X20] mL). The organic phase was dried [(NA2SO4)] and concentrated. The residue was dissolved in hot ethanol (5 mL), and hot water (5 mL) was added to the solution. The product was allowed to crystallize at 0 [C] for 15 h to provide 256 mg (93% yield) of 3-iodocinnamic as pale yellow needles. Mp: [186-188 °C] (lit. 180- [182 °C] See Yuzikhin, [O.] S.; Vasil'ev, A. V.; Rudenko, A. P. [RUSS. J ORG. CLIENI.] 2000, 36, [1743). 1H] NMR (400 MHz, DMSO-d6): 8 12.47 (s, 1H), 8.08 (s, 1H), 7.77 (d, J= 7.8 Hz, 1 H), 7.72 (d, [J=] 7.8 Hz, [1H),] 7.52 (d, [J=] 16.0 Hz, 1 H), 7.22 (t, [J= 7.] 8 Hz, [1H),] 6.5 8 (d, J = 16.0 Hz, 1H). [13C] NMR (100 MHz, DMSO-d6) : 8 167.3, 142.3, 138.6, 136.6, 136.5, 130.9, 127.4, 120.6, 95.4.
References:
WO2004/13094,2004,A2 Location in patent:Page 81
696-41-3
239 suppliers
$7.00/250mg

108-24-7
0 suppliers
$14.00/250ML

41070-12-6
52 suppliers
$182.00/250mg
1664-56-8
5 suppliers
inquiry

41070-12-6
52 suppliers
$182.00/250mg