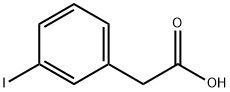
3-Iodophenylacetic acid synthesis
- Product Name:3-Iodophenylacetic acid
- CAS Number:1878-69-9
- Molecular formula:C8H7IO2
- Molecular Weight:262.04
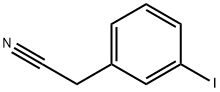
130723-54-5

1878-69-9
General procedure for the synthesis of 3-iodophenylacetic acid from 2-(3-iodophenyl)acetonitrile: 0.2 g of 2-(3-iodophenyl)acetonitrile was dissolved in 1.0 mL of 1.0 M aqueous sodium hydroxide solution and the reaction was carried out at reflux for 4 h. After the reaction was completed, the aqueous phase was extracted with ether. After completion of the reaction, the reaction mixture was extracted with ether and the aqueous phase was acidified with 1.0 M hydrochloric acid solution to pH<7. The acidified aqueous phase was again extracted with ether, and the organic phases were combined, washed with distilled water and dried over anhydrous sodium sulfate. The desiccant was removed by filtration and the filtrate was concentrated under reduced pressure to give 0.17 g of 3-iodophenylacetic acid in 83% yield. The product was characterized by 1H NMR (DMSO-d6): δ 7.65 (s, 1H), 7.5 (d, 1H), 7.3 (d, 1H), 7.1 (m, 1H), 3.6 (s, 2H).

130723-54-5
102 suppliers
$34.00/1g

1878-69-9
178 suppliers
$27.00/1g
Yield:1878-69-9 83%
Reaction Conditions:
with sodium hydroxide in water for 4 h;Heating / reflux;
Steps:
13.1
A solution of 0.2 g of (3-iodophenyl)acetonitrile in 1.0 ml of a 1.0M aqueous sodium hydroxide solution is brought to reflux for 4 hours. The solution is extracted with diethyl ether and the aqueous phase is acidified with a 1.0M hydrochloric acid solution. The solution derived from extracting with diethyl ether is washed with water, dried over sodium sulphate, filtered and then evaporated under reduced pressure to produce 0.17 g of the expected product. Yield: 83% 1H NMR (DMSO) δ (ppm): 7.65 (s, 1H), 7.5 (d, 1H), 7.3 (d, 1H), 7.1 (m, 1H), 3.6 (s, 2H)
References:
Dublanchet, Anne-Claude;Compere, Delphine;Cluzeau, Philippe;Blais, Stephane;Denis, Alexis;Ducrot, Pierre;Courte, Karine;Descamps, Sophie US2004/72871, 2004, A1 Location in patent:Page/Page column 19
201230-82-2
1 suppliers
inquiry
49617-83-6
177 suppliers
$6.00/250mg

1878-69-9
178 suppliers
$27.00/1g
14338-36-4
168 suppliers
$15.00/1g

1878-69-9
178 suppliers
$27.00/1g

49617-83-6
177 suppliers
$6.00/250mg

1878-69-9
178 suppliers
$27.00/1g