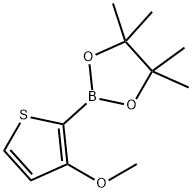
3-Methoxythiophene-2-boronic acid pinacol ester synthesis
- Product Name:3-Methoxythiophene-2-boronic acid pinacol ester
- CAS Number:1310384-98-5
- Molecular formula:C11H17BO3S
- Molecular Weight:240.13
17573-92-1
258 suppliers
$6.00/1g
61676-62-8
323 suppliers
$11.19/5G

1310384-98-5
48 suppliers
$29.00/250mg
Yield:1310384-98-5 43%
Reaction Conditions:
Stage #1: 3-methoxy-thiophenewith n-butyllithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at 20; for 3 h;Inert atmosphere;
Steps:
1 Synthesis of Compound A1
3-methoxythiophene (100 mmol) and tetrahydrofuran (500 mL) was added to a flask, and the reaction system was cooled to -78° C. under a nitrogen atmosphere. Then, n-butyllithium (100 mmol) was slowly added dropwise, and after stirring for one hour, 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (100 mmol) was added. The reaction system was cooled to a room temperature, and stirred for another three hours. Water was added to the reaction system followed by separation, and the obtained crude product was purified by silica gel column chromatography (developing solvent: ethyl acetate) to obtain a compound A1 as a white fine crystal (42.7 mmol, yield: 43%).1H NMR (400 MHz, CDCl3) δ(ppm)=7.47 (d, 1H), 6.89 (d, 1H), 3.91 (s, 3H), 1.34 (s, 12H).
References:
US2019/270758,2019,A1 Location in patent:Paragraph 0107; 0109-0111