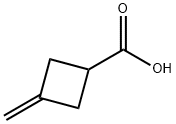
3-METHYLENECYCLOBUTANECARBOXYLIC ACID synthesis
- Product Name:3-METHYLENECYCLOBUTANECARBOXYLIC ACID
- CAS Number:15760-36-8
- Molecular formula:C6H8O2
- Molecular Weight:112.13
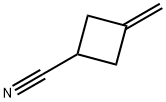
15760-35-7

15760-36-8
Step 1: Preparation of 3-methylenecyclobutanecarboxylic acid Potassium hydroxide (264 g, 4.7 mol) was added to a mixed solution of 3-methylenecyclobutanecarbonitrile (110 g, 1.18 mol) in ethanol (500 mL) and water (500 mL), and the resulting mixture was heated to reflux overnight. Upon completion of the reaction, ethanol was removed by distillation under reduced pressure. The remaining solution was cooled to below 10 °C and acidified with concentrated hydrochloric acid to pH 1. The mixture was extracted with ethyl acetate (2 × 500 mL), the organic phases were combined and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to give the title compound 3-methylenecyclobutanecarboxylic acid (132 g, 100% yield) as a yellow oil.

15760-35-7
141 suppliers
$10.00/1g

15760-36-8
132 suppliers
$18.00/250mg
Yield: 100%
Reaction Conditions:
with water;potassium hydroxide in ethanolReflux;
Steps:
10.1 Step 1:
Preparation of 3-methylidenecyclobutanecarboxylic acid
Step 1:
Preparation of 3-methylidenecyclobutanecarboxylic acid
To a solution of 3-methylidenecyclobutanecarbonitrile (110 g, 1.18 mol) in ethanol (500 mL) and water (500 mL) was added potassium hydroxide (264 g, 4.7 mol) and the resulting mixture was refluxed overnight.
The ethanol was removed under reduced pressure, and then the solution was cooled to below 10° C. and acidified with concentrated HCl to pH 1.
The mixture was extracted with EtOAc (two*500 mL) and the combined organic extracts were dried over anhydrous sodium sulfate and concentrated under vacuum to afford compound the title compound (132 g, 100% yield) as yellow oil.
References:
PFIZER INC.;Cheng, Hengmiao;Johnson, JR., Theodore Otto;Kath, John Charles;Liu, Kevin Kun-Chin;Lunney, Elizabeth Ann;Nagata, Asako;Nair, Sajiv Krishnan;Planken, Simon Paul;Sutton, Scott Channing US2013/79324, 2013, A1 Location in patent:Paragraph 0934; 0935
23761-23-1
483 suppliers
$6.00/1g
2065-66-9
321 suppliers
$5.00/5g

15760-36-8
132 suppliers
$18.00/250mg

463-49-0
53 suppliers
$125.00/10 g
79-10-7
733 suppliers
$20.16/100 mL
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

15760-36-8
132 suppliers
$18.00/250mg

463-49-0
53 suppliers
$125.00/10 g

79-10-7
733 suppliers
$20.16/100 mL

15760-36-8
132 suppliers
$18.00/250mg