3-methylidene-2-oxo-piperidine synthesis
- Product Name:3-methylidene-2-oxo-piperidine
- CAS Number:68074-14-6
- Molecular formula:C6H9NO
- Molecular Weight:111.14
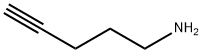
25219-43-6
15 suppliers
inquiry

68074-14-6
14 suppliers
inquiry
Yield:68074-14-6 100%
Reaction Conditions:
with copper(l) iodide;dicyclohexyl-carbodiimide in toluene at 110; for 1.16667 h;
Steps:
14 4.1.14
3-Methylenepiperidin-2-one (30)
4.1.14
3-Methylenepiperidin-2-one (30)
28
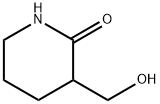
N,N'-Dicyclohexylcarbodiimide (DCC) (346 mg, 1.7 mmol) was added to a stirring solution of alcohol 29 (167 mg, 1.3 mmol) in dry toluene (2 mL).
The mixture was heated at 110 °C (oil bath) and CuI (23 mg, 0.12 mmol) was added and the mixture was stirred for 70 min before it was cooled to room temperature, after which H2O (1.6 mL) was added and stirring continued for 1 h. Et2O (3.4 mL) was added, and the mixture was filtered.
The aqueous phase was separated and extracted four times with 30 mL portions of CH2Cl2.
The combined extracts were dried over K2CO3, filtered and evaporated to give the title compound as a white solid (144 mg, 100%). Rf (EtOAc) = 0.17; 1H NMR (400 MHz, CDCl3): δ = 6.91 (bs, 1H), 6.21 (bs, 1H), 5.37-5.26 (m, 1H), 3.40 (td, J = 6.2, 2.6, 2H), 2.67-2.51 (m, 2H), 1.95-1.79 (m, 2H); 13C NMR (100 MHz, CDCl3): δ = 166.6, 138.1, 122.5, 43.2, 30.4, 23.7; HRMS (ESI): m/z [M+H]+ calcd for C6H10NO: 112.0762; found: 112.0779.
References:
Zachariassen, Zack G.;Thiele, Stefanie;Berg, Erik A.;Rasmussen, Pernille;Fossen, Torgils;Rosenkilde, Mette M.;V?ben?, Jon;Haug, Bengt Erik [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 17,p. 4759 - 4769]
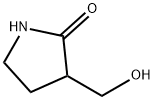
76220-94-5
48 suppliers
$194.03/250mg

68074-14-6
14 suppliers
inquiry
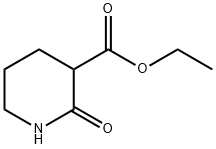
3731-16-6
163 suppliers
$9.00/1g

68074-14-6
14 suppliers
inquiry
![3-[[(methylsulfonyl)oxy]methyl]-2-Piperidinone](/CAS/20180703/GIF/1035042-29-5.gif)
1035042-29-5
6 suppliers
inquiry

68074-14-6
14 suppliers
inquiry