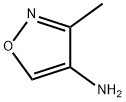
3-METHYLISOXAZOL-4-AMINE synthesis
- Product Name:3-METHYLISOXAZOL-4-AMINE
- CAS Number:354795-62-3
- Molecular formula:C4H6N2O
- Molecular Weight:98.1
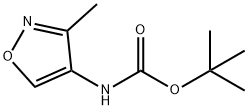
1285517-11-4

354795-62-3
General procedure for the synthesis of 3-methylisoxazol-4-amine from tert-butyl (3-methylisoxazol-4-yl)carbamate: tert-butyl (3-methylisoxazol-4-yl)carbamate (150 mg, 0.75 mmol) was dissolved in a mixture of trifluoroacetic acid (TFA) and dichloromethane (DCM) (10 mL, v/v 1:1), and the reaction was stirred at room temperature for 1 The reaction was stirred for 1 hour at room temperature. After the reaction was completed, the reaction solution was concentrated and the resulting residue was dissolved in dichloromethane (20 mL) and neutralized by adding solid potassium carbonate (K2CO3, 1 g). The solid was removed by filtration and the filtrate was concentrated to give the crude 3-methylisoxazol-4-amine (96 mg, yield: 93%), which could be used in the next reaction without further purification.

1285517-11-4
6 suppliers
inquiry

354795-62-3
35 suppliers
inquiry
Yield:354795-62-3 93%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 1 h;
Steps:
56 Preparation of 3-methylisoxazol-4-amine
[0268j A mixture of tert-butyl (3-methylisoxazol-4-yl)carbamate (150 mg, 0.75 mmol) in TFA/DCM (10 mL, 1:1) was stirred at rt for 1 h. After concentrated, the residue was dissolved in DCM (20 mL) and solid K2C03 (1 g) was added. The solid was filtered off and the filtrate was concentrated to give crude title product (96 mg, yield: 93%), which was used in nest step without further purification.
References:
WO2015/89337,2015,A1 Location in patent:Paragraph 0268
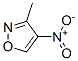
1122-05-0
6 suppliers
inquiry

354795-62-3
35 suppliers
inquiry