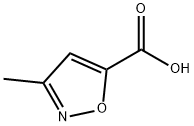
3-METHYLISOXAZOLE-5-CARBOXYLIC ACID synthesis
- Product Name:3-METHYLISOXAZOLE-5-CARBOXYLIC ACID
- CAS Number:4857-42-5
- Molecular formula:C5H5NO3
- Molecular Weight:127.1
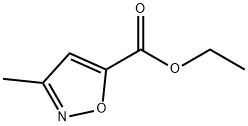
63366-79-0

4857-42-5
General procedure for the synthesis of 3-methylisoxazole-5-carboxylic acid from methyl 3-methyl-5-isoxazolecarboxylate: To a round bottom flask equipped with a magnetic stirrer was added a solution of methyl 3-methyl-5-isoxazolecarboxylate (900 mg, 5.8 mmol) in tetrahydrofuran (2.0 mL). Subsequently, a solution of sodium hydroxide (465 mg, 11.6 mmol) in water (2 mL) was added dropwise to the reaction system and methanol (4 mL) was added. The reaction mixture was stirred at room temperature for 18-20 hours under argon protection. Upon completion of the reaction, the mixture was transferred to a partition funnel and the pH was adjusted to 2 with 1 N hydrochloric acid solution. the aqueous phase was extracted with ethyl acetate (3 x 35 mL), the organic phases were combined and washed with saturated brine (50 mL), dried over anhydrous magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure to afford 3-methylisoxazole-5-carboxylic acid as a white solid (660 mg, 90% yield). The obtained product did not need further purification and could be used directly in the next reaction.

63366-79-0
83 suppliers
$45.00/50mg

4857-42-5
144 suppliers
$17.00/250mg
Yield:4857-42-5 90%
Reaction Conditions:
Stage #1: 3-methylisoxazole-5-carboxylic acid ethyl esterwith sodium hydroxide;water in tetrahydrofuran;methanol at 20; for 18 - 20 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water; pH=2;
Steps:
A round bottom flask with magnetic stirrer was charged with 3-methyl-isoxazole-5- carboxylic acid ethyl ester (900 mg, 5.8 mmol) in tetrahydrofuran (2.0 mL). To the reaction was added a solution of sodium hydroxide (465 mg, 11.6 mmol) in water (2 mL), followed by methanol (4 mL). The reaction was stirred at room temperature for 18 - 20 hours under an argon atmosphere. The reaction was transferred to a separatory funnel and the pH adjusted to 2 via addition of IN hydrochloric acid. The mixture was extracted with ethyl acetate (3 x 35 mL) and the combined extractions were washed with brine (1 x 50 mL), dried over magnesium sulfate, and filtered. The filtrate was concentrated in vacuo to yield S-methyl-isoxazole-S-carboxylic acid as a white solid (660 mg, 90%). The solid was used without purification in the next reaction.
References:
WO2006/91674,2006,A1 Location in patent:Page/Page column 79
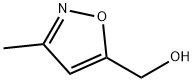
14716-89-3
74 suppliers
$45.00/50mg

4857-42-5
144 suppliers
$17.00/250mg
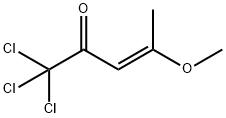
135351-18-7
0 suppliers
inquiry

4857-42-5
144 suppliers
$17.00/250mg
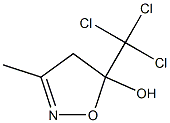
135351-22-3
0 suppliers
inquiry

4857-42-5
144 suppliers
$17.00/250mg
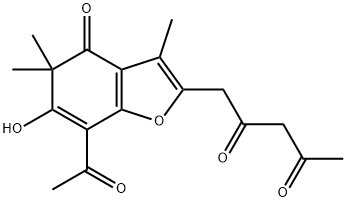
93904-13-3
0 suppliers
inquiry

4857-42-5
144 suppliers
$17.00/250mg