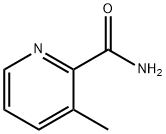
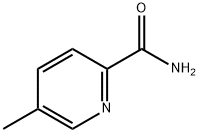
3-Methylpicolinamide synthesis
- Product Name:3-Methylpicolinamide
- CAS Number:937648-82-3
- Molecular formula:C7H8N2O
- Molecular Weight:136.15
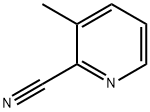
20970-75-6

937648-82-3
General procedure for the synthesis of 3-methyl-2-pyridinecarboxamide from 2-cyano-3-methylpyridine: 2-cyano-3-methylpyridine (2.36 g, 20 mmol) was mixed with concentrated sulfuric acid (12.5 mL), and stirred for 25 min at 80 °C. Upon completion of the reaction, the solution was cooled to room temperature and slowly poured into water (80 mL), which was subsequently neutralized by the addition of saturated aqueous sodium carbonate solution. The reaction mixture was extracted with dichloromethane (3 x 80 mL), the organic layers were combined and dried with anhydrous magnesium sulfate. The organic layer was concentrated under reduced pressure to afford 3-methyl-2-pyridinecarboxamide as a white solid (2.65 g, 97% yield). The product was characterized by 1H NMR (CDCl3, 400 MHz): δ 8.43 (dd, 1H), 7.93 (brs, 1H), 7.62 (dd, 1H), 7.35 (dd, 1H), 5.44 (brs, 1H), 2.76 (s, 3H).

20970-75-6
333 suppliers
$5.00/10g

937648-82-3
50 suppliers
$60.00/100mg
Yield:937648-82-3 97%
Reaction Conditions:
with sulfuric acid at 20 - 80; for 0.416667 h;
Steps:
8 Example 8rac-N-(((2S,3R)-3 -Methyl-i -(2-methyl-S -phenylthiazole-4-carbonyl)piperidin-2- ylmethyliuinoline-8-carboxamide
Example 8rac-N-(((2S,3R)-3 -Methyl-i -(2-methyl-S -phenylthiazole-4-carbonyl)piperidin-2- ylmethyliuinoline-8-carboxamide3 -MethylpicolinamideLN_I,NH20A mixture of 3-methylpicolinonitrile (2.36 g, 20 mmol) and conc. sulfuric acid (12.5mL) was stirred at 80°C for 25 mm. The solution was cooled to r.t., poured into water (80mL), followed by addition of sat. Na2CO3 (aq.) until pH 7. The resulting mixture was extracted with DCM (3x) and the combined organic layer was dried over Mg504 and concentrated in vacuo to provide 3-methylpicolinamide as a white solid (2.65 g, 97%). ‘H74NMR (CDC13, 400 MHz) ? 8.43 (dd, 1H), 7.93 (brs, 1H), 7.62 (dd, 1H), 7.35 (dd, 1H), 5.44 (brs, 1H), 2.76 (s, 3H).
References:
WO2013/119639,2013,A1 Location in patent:Page/Page column 74; 75
108-99-6
338 suppliers
$9.00/5g
60100-09-6
0 suppliers
inquiry
20970-77-8
17 suppliers
inquiry

937648-82-3
50 suppliers
$60.00/100mg