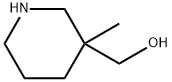
(3-METHYLPIPERIDIN-3-YL)METHANOL synthesis
- Product Name:(3-METHYLPIPERIDIN-3-YL)METHANOL
- CAS Number:221298-00-6
- Molecular formula:C7H15NO
- Molecular Weight:129.2
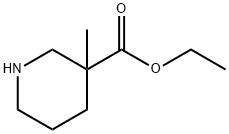
170843-43-3
50 suppliers
inquiry

221298-00-6
24 suppliers
$90.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 3-methylpiperidine-3-carboxylic acid ethyl esterwith lithium aluminium tetrahydride in tetrahydrofuran at 18 - 25; for 15 h;
Stage #2: with sodium hydroxide;ammonia;water;Rochelle's salt in tetrahydrofuran at -5; for 0.5 h;
Steps:
17.1
EXAMPLE 173-(4-Methylsulfonyl)phenyl-2-(3-hydroxymethyl-3-methyl)piperidin-1-yl-5-trifluoromethylpyridineStep 1: 3-Hydroxymethyl-3-methylpiperidine To ethyl nipecotate (1.3 g) in THF (20 mL) at r.t was added potassium bis(trimethylsilyl)amide (18 mL of a 0.5 M solution in toluene). After 1 h, methyl iodide (0.5 mL) was added and the mixture was stirred for 15 h. Water was added and the mixture extracted with ether. The organics were washed with brine, dried and concentrated. The residual material (1.3 g) was dissolved in THF (20 mL) and treated with lithium aluminum hydride (8.7 mL of a 1 M solution in THF) at r.t. After 15 h, the mixture was cooled to -5°C and aqueous sodium potassium tartrate was added followed by concentrated NH4OH, 10 N NaOH and the resulting mixture was stirred for 30 min. The mixture was filtered through anhydrous sodium sulfate and the filtrate was concentrated from toluene twice. The residual oil (1 g), containing the title compound, was used without further purification in Step 2.
References:
EP1015431,2005,B1 Location in patent:Page/Page column 28