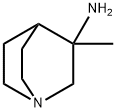
3-Methylquinuclidin-3-aMine hydrobroMide synthesis
- Product Name:3-Methylquinuclidin-3-aMine hydrobroMide
- CAS Number:21638-13-1
- Molecular formula:C8H16N2
- Molecular Weight:140.23
![Acetamide, N-(3-methyl-1-azabicyclo[2.2.2]oct-3-yl)-](/CAS/20210305/GIF/21638-06-2.gif)
21638-06-2
0 suppliers
inquiry

21638-13-1
12 suppliers
inquiry
Yield:21638-13-1 69%
Reaction Conditions:
with hydrogenchloride for 72 h;Reflux;
Steps:
A well-stirred 3.0 M solution of methyllithium in diethyl ether (67.0 mL, 201 mmol) was diluted with additional diethyl ether (150 mL), cooled to -78 °C and treated, dropwise, with a solution of quinuclidin-3-one (12.5 g, 100 mmol) in diethyl ether (100 mL). The resulting solution was maintained at -78 °C for 1 hour and then allowed to warm to room temperature. After overnight stirring, the reaction was recooled (0 °C) and treated, dropwise, with water (60 mL). The mixture was concentrated and the resulting residue was purified by flash chromatography over neutral aluminum oxide using a chloroform/methanol gradient (0-20% methanol) to give 3-methylquinuclidin-3-ol (10.0 g, 71%o) as a light yellow solid. To stirred acetonitrile (250 mL) at 0 °C, was slowly added concentrated sulfuric acid (100 mL). The resulting solution was added dropwise to a mixture of 3-methylquinuclidin-3-ol (9.10 g, 64.5 mmol) in acetonitrile (250 mL) at 0 °C. The reaction mixture was stirred at room temperature for 60 hours, cooled to (0 °C) and then basified (pH -10) with aqueous sodium hydroxide solution. The mixture was extracted with 5: 1 (v/v) chloroform/isopropanol. The combined organic layers were concentrated and the residue was diluted with 2N hydrochloric acid. After washing with 5: 1 (v/v) chloroform/isopropanol, the aqueous layer was basified with 2N aqueous sodium hydroxide and extracted with 5: 1 (v/v) chloroform/isopropanol. The combined organic layers were washed with water, dried (Na2S04) and concentrated to afford N-(3- methylquinuclidin-3-yl)acetamide as a light yellow oil (9.50 g, 82%). A solution of the above acetamide intermediate (9.50 g, 52.0 mmol) in concentrated hydrochloric acid (100 mL) was refluxed for 3 days. After cooling in an ice bath, the reaction was treated with enough aqueous sodium hydroxide solution to achieve pH ~1. The mixture was washed with 5: 1 (v/v) chloroform/isopropanol. The aqueous layer was then basified with 2N aqueous sodium hydroxide solution and extracted with 5: 1 (v/v) chloroform/isopropanol. The combined extracts were washed with water, dried (Na2S04) and concentrated to afford the title compound as a light yellow semi- solid (5.00 g, 69%). 1H NMR (500 MHz, DMSO-^) d 2.72-2.39 (m, 6H), 2.01-1.96 (m, 1H), 1.67-1.61 (m, 1H), 1.43-1.36 (m, 2H), 1.23-1.17 (m, 1H), 1.09 (s, 3H) ppm. 13C NMR (125 MHz, DMSO-^) d 65.3, 48.3, 46.6, 46.4, 34.2, 30.0, 24.8, 22.8 ppm.
References:
WO2014/43068,2014,A1 Location in patent:Page/Page column 163; 164
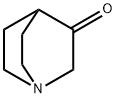
3731-38-2
84 suppliers
inquiry

21638-13-1
12 suppliers
inquiry

16283-66-2
8 suppliers
inquiry

21638-13-1
12 suppliers
inquiry