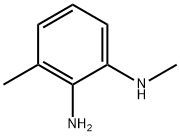
3,N*1*-Dimethyl-benzene-1,2-diamine synthesis
- Product Name:3,N*1*-Dimethyl-benzene-1,2-diamine
- CAS Number:73902-64-4
- Molecular formula:C8H12N2
- Molecular Weight:136.19
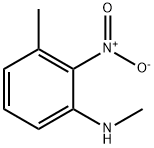
70254-75-0

73902-64-4
N,3-dimethyl-2-nitroaniline (160 mg, 0.96 mmol) was used as raw material, which was dissolved in methanol (10 mL) and 10% Pd/C (160 mg) was added. The reaction mixture was stirred for 2 h at room temperature under hydrogen atmosphere. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the reaction mixture was filtered through a bed of diatomaceous earth and washed with methanol (50 mL). The filtrate was concentrated under vacuum to give N1,3-dimethylbenzene-1,2-diamine as a red liquid. Yield: 100 mg (82%).1H NMR (400 MHz, DMSO-d6) δ: 6.70-6.81 (m, 1H), 6.58-6.66 (m, 2H), 2.87 (s, 3H), 2.21 (s, 3H). Mass spectrum (ESI+) m/z: 137.01 [M + H]+.

70254-75-0
7 suppliers
inquiry

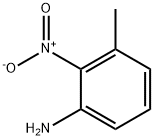
73902-64-4
27 suppliers
$146.00/100mg
Yield:73902-64-4 82%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen at 20;
Steps:
N1,3-Dimethylbenzene-1 ,2-diamine
To a solution of N,3-dimethyl-2-nitroaniline (160mg, 0.96mmol) in MeOH (lOmL), 10% Pd-C (160 mg) was added . The reaction mixture was stirred at rt under H2 balloon atmosphere for 2 h. TLC showed the reaction to be complete and filtered through a celite bed and washed with MeOH (5OmL). The filtrate was evaporated under vacuum to afford N1,3-dimethylbenzene-1,2-diamine as a red liquid. Yield:100mg (82%); 1H NMR (400 MHz; DMSO-d6): 6.70-6.81 (m, 1H), 6.58-6.66 (m, 2H), 2.87 (5, 3H), 2.21 (5, 3H); MS (ESl+) for CHNOS m/z 137.01 [M+H].
References:
WO2018/37223,2018,A1 Location in patent:Page/Page column 120; 121
601-87-6
115 suppliers
inquiry

73902-64-4
27 suppliers
$146.00/100mg

90868-29-4
2 suppliers
inquiry

73902-64-4
27 suppliers
$146.00/100mg
3013-27-2
138 suppliers
$10.00/1g

73902-64-4
27 suppliers
$146.00/100mg