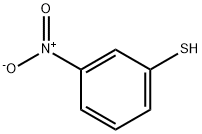
3-Nitro-benzenethiol synthesis
- Product Name:3-Nitro-benzenethiol
- CAS Number:3814-18-4
- Molecular formula:C6H5NO2S
- Molecular Weight:155.17
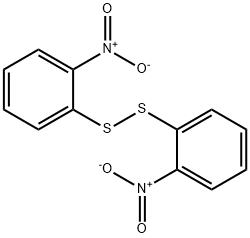
1155-00-6
164 suppliers
$45.89/25g

3814-18-4
18 suppliers
inquiry
Yield: 83%
Reaction Conditions:
Stage #1:bis(2-nitrophenyl)disulfide with sodium tetrahydroborate in tetrahydrofuran at 20; for 2 h;
Stage #2: with water in tetrahydrofuran at 0;
Steps:
m-Nitrobenzenethiol (43) (Cashman, J. R. et al. Chem. Res. Toxicol., 1989, 2:392-399). To a solution of m-nitrobenzene disulfide (300 mg, 0.97 mmol) in anhydrous THF (2 mL) was added solid NaBH4 (140 mg, 3.4 mmol) in small portions. The resulting mixture was stirred at room temperature under an inert atmosphere. After 2 hr, the reaction mixture was cooled in an ice bath, and then about 5 mL of ice water was added to the mixture. The resulting mixture was acidified with HCl (1 M), then extracted with CH2Cl2 (10 mL). The organic layer was then washed with water (10 mL), then brine (10 mL), dried over MgSO4, and concentrated in vacuo to give 43 (248 mg, 83%) as a pale yellow oil. 1H NMR (CDCl3, 400 MHz): δ 8.10 (d, J=2.0 Hz, 1H); 7.97 (dd, J=8.0, 2.0 Hz, 1H); 7.54 (d, J=8.0 Hz, 1H); 7.38 (t, J=8.0 Hz, 1H); 3.68 (s, 1H); 13C NMR (CDCl3, 100 MHz): δ 134.9, 130.0=, 123.9, 120.7.
References:
Turos, Edward;Revell, Kevin D. US2008/182815, 2008, A1 Location in patent:Page/Page column 9
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3814-18-4
18 suppliers
inquiry
645-00-1
15 suppliers
$45.39/25g

3814-18-4
18 suppliers
inquiry
537-91-7
130 suppliers
$14.14/1gm:

3814-18-4
18 suppliers
inquiry
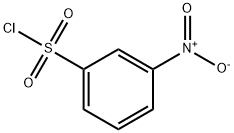
121-51-7
16 suppliers
$15.00/5 g

3814-18-4
18 suppliers
inquiry