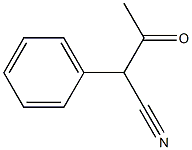
3-oxo-2-phenylbutanenitrile synthesis
- Product Name:3-oxo-2-phenylbutanenitrile
- CAS Number:57115-24-9
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
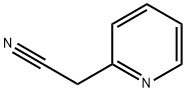
2739-97-1

75-36-5

57115-24-9
The general procedure for the synthesis of 3-oxo-2-(pyridin-2-yl)butanenitrile from 2-pyridylacetonitrile and acetyl chloride was as follows: to a solution of 2-(2-pyridyl)acetonitrile (2.00 g, 16.93 mmol, 1.83 mL) in tetrahydrofuran (THF, 5 mL) was added sequentially sodium ethoxide (EtONa, 3.46 g, 50.79 mmol, 3.00 eq. ) and acetyl chloride (2.18 g, 33.86 mmol, 2.00 equiv). The reaction mixture was stirred at 10°C for 16 hours. The progress of the reaction was monitored by thin layer chromatography (TLC, unfolding agent: ethyl acetate), showing about 40% of the raw material remaining and the formation of a new major spot. Upon completion of the reaction, the mixture was diluted with water (50 mL) and adjusted with saturated aqueous sodium bicarbonate (NaHCO3) to pH=5. Subsequently, the reaction mixture was extracted with ethyl acetate (50 mL x 3). The organic layers were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (eluent: 5-50% ethyl acetate/petroleum ether) to afford 3-oxo-2-(2-pyridyl)butanenitrile (780 mg, 4.87 mmol, 29% yield) as a yellow solid. The structure of the product was confirmed by liquid chromatography-mass spectrometry (LC-MS, ESI), m/z 161.0 [M+H]+.
Yield:57115-24-9 29%
Reaction Conditions:
with sodium ethanolate in tetrahydrofuran at 10; for 16 h;
Steps:
11.a Step a: Synthesis of 3-oxo-2-(2-pyridyl)butanenitrile
[507] To a solution of 2-(2-pyridyl)acetonitrile (2,00 g, 16,93 mmol, 1.83 mL, 1 ,00 eg) in THF (5 mL) was added EtONa (3.46 g, 50.79 mmol, 3.00 eg) and formyl chloride (2.18 g, 33.86 mmol, 2.00 eg). The reaction mixture was stirred at 10 °C for 16 h. TLC (ethyl acetate) indicated -40% of SM remained and one major new spot formed. The reaction mixture was diluted with water (50 mL), and adjusted to pH=5 with sat. aq. KHSO4 solution. Then the resulting mixture was extracted with ethyl acetate (50 mL χ 3). The organic layers were combined, dried over anhydrous NazSC , filtered and concentrated under reduced pressure to give a residue, which was purified by column chromatography on silica gel (5- 50% ethyl acetate/petroleum ether) to afford 3-oxo-2-(2-pyridyl)butanenitrile (780 mg, 4.87 mmol, 29% yield) as a yellow solid. LC-MS (ESI): m/z 161.0 { .M 1 I s ' .
References:
WO2017/210678,2017,A1 Location in patent:Paragraph 506; 507