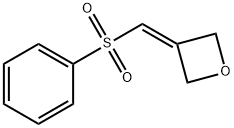
3-(phenylsulfonylMethylene)oxetane synthesis
- Product Name:3-(phenylsulfonylMethylene)oxetane
- CAS Number:1221819-46-0
- Molecular formula:C10H10O3S
- Molecular Weight:210.25
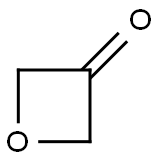
6704-31-0
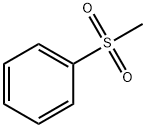
3112-85-4

1221819-46-0
General procedure: n-Butyl lithium (n-BuLi, 2.5 M hexane solution, 12.2 mL, 30.6 mmol) was slowly added dropwise to a solution of (methylsulfonyl)benzene (2.2 g, 13.9 mmol) in tetrahydrofuran (THF, 38 mL) at 0 °C, with the dropwise addition time being controlled at 10 minutes. After the dropwise addition, the reaction mixture was continued to be stirred for 30 minutes. Subsequently, chlorodiethylphosphonate (2.4 mL, 16.7 mmol) was added dropwise to the reaction system.After 30 min, oxetan-3-one (1.0 g, 13.9 mmol) was dissolved in THF (2 mL) and slowly added dropwise to the reaction mixture at -78 °C. After completion of the dropwise addition, the reaction mixture was stirred continuously at -78 °C for 2 hours. At the end of the reaction, the reaction mixture was diluted with aqueous ammonium chloride (NH4Cl) (100 mL) and extracted with ethyl acetate (EtOAc, 100 mL x 2). The organic layers were combined, concentrated and the residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate=3/1) to afford the target product 3-((phenylsulfonyl)methylene)oxetane (2.4 g, 82% yield) as a colorless oil.1H NMR (400 MHz, CDCl3): δ 7.90-7.88 (m, 2H), 7.68- 7.64 (m, 1H), 7.57 (t, J=7.6Hz, 2H), 6.13-6.11 (m, 1H), 5.66-5.64 (m, 2H), 5.30-5.27 (m, 2H).

6704-31-0
320 suppliers
$6.00/1g

3112-85-4
208 suppliers
$15.00/5g

1221819-46-0
54 suppliers
$30.00/100mg
Yield:1221819-46-0 82%
Reaction Conditions:
Stage #1:methylphenylsulfonate with n-butyllithium in tetrahydrofuran;hexane at 0; for 0.666667 h;
Stage #2: with diethyl chlorophosphate in tetrahydrofuran;hexane for 0.5 h;
Stage #3:oxetan-3-one in tetrahydrofuran;hexane at -78; for 2 h;
Steps:
A-9 3-((Phenylsulfonyl)methylene)oxetane (D A-9)
To a solution of (methylsulfonyl)benzene (2.2 g, 13.9 mmol) in THF (38 mL) at 0 was added n-BuLi (2.5 M in hexanes, 12.2 mL, 30.6 mmol) dropwise over 10 minutes. After the mixture was stirred for 30 min, chlorodiethylphosphonate (2.4 mL, 16.7 mmol) was added dropwise to the reaction. After 30 minutes, a solution of oxetan-3-one (1.0 g, 13.9 mmol) in THF (2 mL) was added dropwise to the reaction mixture at -78 . The reaction mixture was stirred at -78 for 2 hours, then diluted with aqueous NH4Cl (100 mL) and extracted with EtOAc (100 mL x 2) . The combined organic layers were concentrated and the residue was purified by silica gel chromatxography column (petroleum ether/EtOAc = 3/1) to give the title compound (2.4 g, 82%) as a colorless oil. 1H NMR (400 MHz, CDCl3) : δ7.90-7.88 (m, 2H) , 7.68-7.64 (m, 1H) , 7.57 (t, J= 7.6 Hz, 2H) , 6.13-6.11 (m, 1H) , 5.66-5.64 (m, 2H) , 5.30-5.27 (m, 2H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;GLAXOSMITHKLINE (CHINA) R&D COMPANY LIMITED;REN, Feng;SANG, Yingxia;XING, Weiqiang;ZHAN, Yang;ZHAO, Baowei WO2018/137619, 2018, A1 Location in patent:Page/Page column 54; 55

6704-31-0
320 suppliers
$6.00/1g
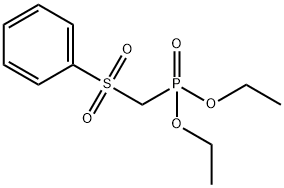
56069-39-7
96 suppliers
inquiry

1221819-46-0
54 suppliers
$30.00/100mg