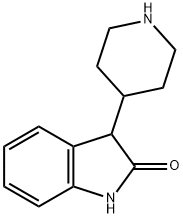
3-(piperidin-4-yl)indolin-2-one synthesis
- Product Name:3-(piperidin-4-yl)indolin-2-one
- CAS Number:72831-89-1
- Molecular formula:C13H16N2O
- Molecular Weight:216.28
![2H-Indol-2-one, 1,3-dihydro-3-[1-(phenylmethyl)-4-piperidinylidene]-](/CAS/20210305/GIF/72831-92-6.gif)
72831-92-6
2 suppliers
inquiry

72831-89-1
31 suppliers
inquiry
Yield:72831-89-1 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;acetic acid in methanol at 50; under 2585.81 Torr; for 3 h;
Steps:
55.2 Step 2. Synthesis of 3-(piperidinbut-4-yl)indol-2-one (GEN1-051-2)
3-(1-benzyl-4-piperidinyl)indol-2-one (200 mg, 0.637 mmol) and Pd/C (10%, 100 mg) were added to a solution of AcOH (0.1 mL) and MeOH (5 mL), and it was stirred at 50°C under H2 (50Psi) for 3 hours.
The mixture was filtered and the filtrate was concentrated.
The residue was dissolved in MeOH (1 mL), and 10% NaOH aqueous solution was added to adjust pH=8-9.
The mixture was concentrated and triturated with DCM/MeOH (10/1, 30 mL) and filtered.
The filtrate was concentrated to obtain the desired compound (140 mg, yield 98%) as a yellow oil. MS (ESI): Rt = 0.56 min, m/z 217.2 [M+H]+; Purity: 100% 254 nm, 100% 214 nm.
References:
EP3960736,2022,A1 Location in patent:Paragraph 0755; 0758-0759
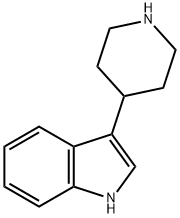
17403-09-7
123 suppliers
$29.00/100mg

72831-89-1
31 suppliers
inquiry
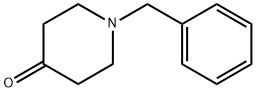
3612-20-2
452 suppliers
$6.00/10g

72831-89-1
31 suppliers
inquiry
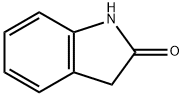
59-48-3
481 suppliers
$5.00/1g

72831-89-1
31 suppliers
inquiry