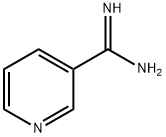
3-PYRIDINECARBOXAMIDINE synthesis
- Product Name:3-PYRIDINECARBOXAMIDINE
- CAS Number:23255-20-1
- Molecular formula:C6H7N3
- Molecular Weight:121.14
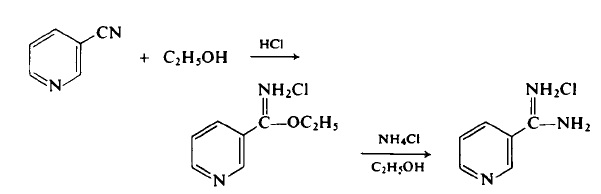
100-54-9
569 suppliers
$5.00/10g

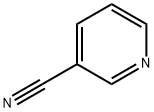
23255-20-1
83 suppliers
$110.00/100mg
Yield:-
Reaction Conditions:
with hydrogenchloride;ethanol;chloroformErwaermen des Reaktionsprodukts in wss. Aethanol mit NH4Cl;
References:
Barber;Slack [Journal of the American Chemical Society,1944,vol. 66,p. 1607]