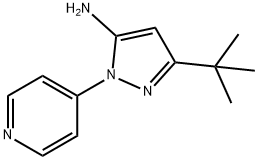
3-(tert-Butyl)-1-(pyridin-4-yl)-1H-pyrazol-5-amine synthesis
- Product Name:3-(tert-Butyl)-1-(pyridin-4-yl)-1H-pyrazol-5-amine
- CAS Number:884340-12-9
- Molecular formula:C12H16N4
- Molecular Weight:216.28
Yield:884340-12-9 92%
Reaction Conditions:
in ethanol;Heating / reflux;
Steps:
16.2
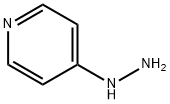
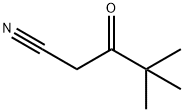
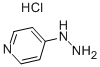
A solution of 4-hydrazinopyridine (2.4g, 16.9 mmol) and 4,4-dimethyl-3-oxo-pentanenitrile (2.32 g, 18.1 mmol) in ethanol (25 ml_) was heated to reflux overnight. The reaction mixture was cooled to rt and then concentrated under reduced pressure. The residue was purified using silica gel chromatography (eluent: 30% ethyl acetate in hexanes) to provide the title compound (3.84 g, 92%). 1H NMR (400 MHz, DMSO-d6) δ 8.8 (s, 2H), 8.2 (s, 2 H), 5.96-6.06 (bs, 2 H), 5.6 (s, 1 H), 1.19 (s, 9 H).; ES-MS m/z 217.2 [M+Hf, LCMS RT (min) 1.94.
References:
WO2007/64872,2007,A2 Location in patent:Page/Page column 83
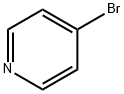
1120-87-2
167 suppliers
inquiry

884340-12-9
8 suppliers
inquiry
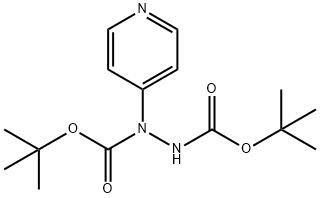
614717-75-8
0 suppliers
inquiry

884340-12-9
8 suppliers
inquiry